The Hayward, California-based company completed the first five procedures in its first-in-human feasibility study. Treating physicians successfully discharged all patients. They’ll continue to be monitored and evaluated over the coming months to assess safety and effectiveness. Pulse Biosciences plans to evaluate the primary safety endpoint at 30 days.
Dr. Vivek Reddy of Mount Sinai Hospital (New York) and Dr. Petr Neuzil of Na Homolce Hospital, Prague, and colleagues performed the procedures. They used the CellFX nxPFA (pulsed-field ablation) 360 catheter integrated with the CardioNXT iMap mapping and navigation. The doctors successfully treated five patients with AFib at the Czech Republic hospital.
“We have been collaborating with Pulse Biosciences to bring their novel nsPFA technology to the clinical realm, and are excited to report that our experience with these first five patients has validated our belief that this may represent the next generation of PFA technology for the treatment of AF,” said Dr. Reddy. “The results were consistent with our preclinical experience. Importantly, the speed and ease with which we were able to isolate the pulmonary veins with the nsPFA 360 catheter was impressive and all patients tolerated the procedure well. Now we look forward to completing enrollment in this study to fully assess the safety and durability of nsPFA treatment.”
More on Pulse Biosciences’ technology and the study
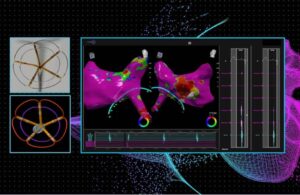
Pulse Biosciences designed its investigational CellFX nsPFA 360 cardiac catheter to produce a nonthermal ablation. The company targets pulmonary vein isolation with the proprietary energy to treat AFib.
According to Pulse, it designed the catheter to deliver a fast, transmural and fully circumferential ablation in a single energy delivery. The catheter features 3D mapping and navigation for comprehensive visualization and precise ablation.
Pulse Biosciences plans to enroll up to 30 patients in the current feasibility study. It expects more updates on the first-in-human procedures in the coming months. The company also anticipates initiating the regulatory process with the FDA and other worldwide regulatory authorities in the coming quarters.
The company says it aims to push the boundaries of possibilities in bioelectric energy. President and COE Kevin Danahy believes it could reshape the future of AFib treatment.
“As we embark on this new era of advancement in medical device technology, our mission is clear: to revolutionize healthcare with CellFX nsPFA, with the intention of significantly improving clinical outcomes for both patients and physicians,” Danahy said. “CellFX nsPFA will empower doctors, inspire patients to seek life-altering treatment, and create a world where CellFX nsPFA technology can become a catalyst for healing and hope.”

