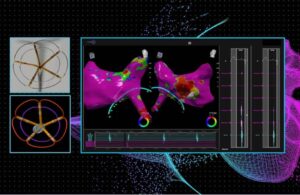
Pulse Biosciences (Nasdaq:PLSE) today announced the completion of the first-in-human procedures for its CellFX nsPFA cardiac catheter. The Hayward, California-based company completed the first five procedures in its first-in-human feasibility study. Treating physicians successfully discharged all patients. They'll continue to be monitored and … [Read more...] about Pulse Biosciences announces first human use of pulsed-field ablation catheter
Pulse Biosciences
Pulse Biosciences, CardioNXT to collab on pulsed-field ablation trial
Pulse Biosciences (Nasdaq:PLSE) announced today that it entered into a collaboration with CardioNXT to support its AFib treatment. The collaboration supports Pulse Biosciences' planned first-in-human study of its pulsed-field ablation technology. Hayward, California-based Pulse develops a proprietary nanosecond pulsed-field ablation (nsPFA) … [Read more...] about Pulse Biosciences, CardioNXT to collab on pulsed-field ablation trial