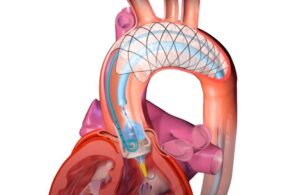
EnCompass Technologies announced today that it received FDA investigational device exemption for its F2 cerebral embolic protection system. The conditional IDE enables a study of the F2 system for protecting patients from brain injury during cardiovascular procedures. According to a news release, all cardiovascular procedures cause the … [Read more...] about FDA grants IDE to EnCompass for cerebral embolic protection system in TAVR procedures