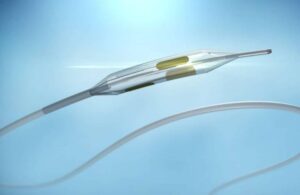
Metavention announced today that the FDA granted approval to initiate an investigational device exemption (IDE) trial for its renal denervation system. Minneapolis-based Metavention designed its integrated radiofrequency (iRF) renal denervation system to treat hypertension. The company plans for its randomized, double-blinded, sham-controlled … [Read more...] about FDA approves Metavention’s renal denervation for hypertension pivotal IDE study