Olympus announced today that it made a voluntary corrective action to provide further clarification on the use of certain bronchoscopes. The company's global action aims to outline the safe and effective use of its bronchoscopes with laser therapy, argon plasma coagulation or high-frequency cauterization equipment during therapeutic procedures … [Read more...] about Olympus issues label update for certain bronchoscopes related to endobronchial combustion
Endoscopes
Gore, Olympus ink distro deal for Viabil endoprosthesis
Olympus announced today that it entered into a distribution agreement with W.L. Gore & Associates for the Gore Viabil biliary endoprosthesis. The international agreement makes Olympus the exclusive distributor for the Viabil endoprosthesis for endoscopic placement. Olympus plans to begin commercialization over the coming months, beginning in … [Read more...] about Gore, Olympus ink distro deal for Viabil endoprosthesis
Outlook Surgical wins FDA clearance for hybrid endoscope system
Outlook Surgical this week announced it received FDA 510(k) clearance for its Inova 1 Towerless Endoscope System. The Bloomington, Illinois-based company designed the device to combine the features of a rigid and flexible scope into a single platform. It is intended for head and neck procedures, including ENT and related specialties. Outlook … [Read more...] about Outlook Surgical wins FDA clearance for hybrid endoscope system
Zenflow gets FDA nod for single-use cystoscope
Zenflow announced today that it received FDA 510(k) clearance for its Spring scope and camera control unit (CCU). The South San Francisco, California-based company touts its Spring scope and CCU as the first single-use cystoscope of its kind. It features a 12-fr working channel, making it more than 80% larger than current single-use models, the … [Read more...] about Zenflow gets FDA nod for single-use cystoscope
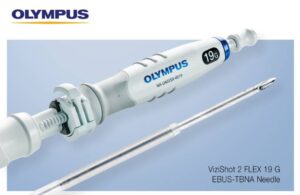
Olympus issues voluntary recall for certain endoscope needles
Olympus announced a voluntary, global medical device removal of certain ViziShot 2 FLEX (19G) EBUS -TBNA (ViziShot 2 FLEX) needles. The company manufactured affected needles prior to May 12, 2025. It initiated the recall after receiving reports of device components detaching during procedures. Olympus said that it assessed the issue after … [Read more...] about Olympus issues voluntary recall for certain endoscope needles
Dornier MedTech launches new ureteral access sheath and ureteroscope in U.S.
Dornier MedTech America announced the full U.S. commercial launch of two single-use devices, the Dornier Hoover Flexible and Navigable Suction Ureteral Access Sheath and the Dornier Axis II Slim ureteroscope. Both devices had already received FDA clearance and were in limited release before the full U.S. launch. The Hoover sheath provides … [Read more...] about Dornier MedTech launches new ureteral access sheath and ureteroscope in U.S.
Olympus enters distro deal with MacroLux for single-use urology products
Olympus announced an exclusive global distribution deal with MacroLux Medical Technology to distribute single-use urology products. The agreement includes multiple single-use cytoscopes, ureteroscopes and suction access sheaths. These devices offer access and visualization in the bladder and urinary tract. They enable the diagnosis and treatment … [Read more...] about Olympus enters distro deal with MacroLux for single-use urology products
Pavmed licenses endoscopic esophageal imaging tech out of Duke
Pavmed announced today that it executed a non-binding letter of intent to license endoscopic esophageal imaging technology from Duke University. Through a newly formed subsidiary, Pavmed licensed the technology designed to identify and facilitate treatment of advanced esophageal precancer (dysplasia) during upper endoscopy. It features a … [Read more...] about Pavmed licenses endoscopic esophageal imaging tech out of Duke
EvoEndo earns expanded FDA clearance for gastroscope
EvoEndo announced today that it received expanded FDA clearance for its Model LE gastroscope, covering patients of all ages. Denver-based EvoEndo's offering now covers neonates to adults, expanding the previous indication for patients aged five and older. The company says its system has been the only option available for sedation-free transnasal … [Read more...] about EvoEndo earns expanded FDA clearance for gastroscope
FDA wants to prevent import of certain Olympus scopes manufactured in Japan
The FDA is notifying healthcare providers about import alerts for certain medical devices manufactured in Japan by Olympus and its subsidiaries. According to a notice, the FDA has "concerns related to outstanding quality system regulation violations by Olympus." The agency cites "extensive and ongoing efforts to address compliance issues" with the … [Read more...] about FDA wants to prevent import of certain Olympus scopes manufactured in Japan