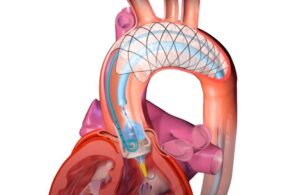
The conditional IDE enables a study of the F2 system for protecting patients from brain injury during cardiovascular procedures.
According to a news release, all cardiovascular procedures cause the release of particulate debris and air bubbles — emboli. Emboli can cause injury if they reach the brain. This can occur in procedures like transcatheter aortic valve replacement (TAVR), an ever-growing cardiovascular procedure.
EnCompass’ F2 filter has pores small enough to block most emboli to the brain while preserving blood flow. During the TAVR procedure, 360-degree wall apposition of the filter in the aortic arch prevents migration. The filter, attached to a self-expanding, nitinol stent, is easy to insert, deploy and retrieve, according to the company.
Earlier this year, researchers presented results from an in vitro study comparing F2 versus Boston Scientific’s Sentinel. EnCompass said that, for all sizes of emboli, the F2 filter prevented 94% more emboli from reaching the brain than Sentinel. The company began a first-in-human study in Europe for three patients undergoing TAVR. The small cohort demonstrated 100% procedural success and no adverse events.
A similar pilot study began this month in Australia, and with the IDE, the company plans to commence its first U.S. pilot study. It expects those to lead to a U.S. pivotal clinical trial in 2024.
“Brain injury after TAVR, as well as other cardiovascular procedures, remains a significant problem. As most of these complications are caused by emboli, it makes sense to use a filter that blocks most harmful emboli from entering the brain,” said George Wallace, CEO of EnCompass Technologies. “The US pilot study, along with our growing clinical experience outside the US, represents the beginning of our clinical journey towards demonstrating meaningful patient benefits.”
