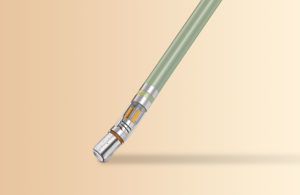
Johnson & Johnson (NYSE:JNJ) recently announced that its Biosense Webster business has secured FDA approval for its Thermocool SmartTouch SF ablation catheter.
The Thermocool SmartTouch received approval for the treatment of persistent atrial fibrillation, J&J announced yesterday.
The approval came after results from the 381-patient, prospective, multi-center Precept study that found that 80% of persistent AF patients experienced clinical success at 15 months after ablation therapy using the Thermocool SmartTouch SF catheter with Carto Visitag module.
The study also found people suffering from AF experienced a clinically meaningful improvement in their quality of life, with a significant reduction in healthcare resource utilization post-ablation.
“Every patient and every arrhythmia are unique,” said Dr. Francis Marchlinski, director of electrophysiology at the University of Pennsylvania Health System. “This approval and the Precept data provide evidence to support a tailored approach using the Carto 3 System and Thermocool SmartTouch SF Catheter to treat persistent AF patients, who are more at risk for stroke and other complications from their AF.”
Uri Yaron, worldwide president of Biosense Webster, added that persistent AF patients face a higher risk of complications such as stroke, heart failure, and death. “This approval and data from the Precept study help to further our commitment to advancing AF treatment, providing electrophysiologists with state-of-the-art options for their patients.”

