The regulatory nod in Japan further expands the reach of the system, which in December became the first PFA system approved in the United States to treat paroxysmal and persistent atrial fibrillation (AFib). Since then, Boston Scientific’s Farapulse system won FDA approval to treat paroxysmal AFib, where the heart’s beating returns to normal within seven days, with plans to seek approval for persistent AFib.
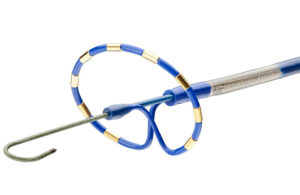
Pulsed field ablation has generated a great deal of excitement in the medical device industry. Its potential advantages versus radiofrequency ablation or cryoablation include the characteristic that heart muscle tissue can be especially susceptible to it, while other types of surrounding tissue are injury-resistant.
More than 250 physicians worldwide have successfully treated more than 3,000 patients with PulseSelect — together with the company’s bi-directional 10F FlexCath Contour sheath, according to Medtronic.
Japanese Ministry of Health, Labour and Welfare approval came after the pivotal PULSED AF trial, a global, multi-center IDE study that included centers in Japan.
“We are seeing tremendous excitement and adoption of PulseSelect in every market we have launched, including the U.S., Europe and Canada. The positive feedback on safety profile, ease of handling, and its flexibility to be used with any mapping system has been consistent across many focal RF and single-shot shot users who have adopted it,” Rebecca Seidel, president of Medtronic’s Cardiac Ablation Solutions business, said in a news release.
“And now the expansion of PulseSelect into the important Japanese market is exciting for both physicians and for the patients they serve, who deserve the most advanced, safe, effective, and efficient care for AFib.”
Also today, Medtronic said that it has a new software upgrade available for PulseSelect that includes an automated delivery mode. The new automated delivery mode is meant to further simplify the workflow and provide additional options for physicians to control PFA during the procedure. Medtronic has completed upgrades across existing and new systems in Europe and Canada. The upgrades have now been initiated in the U.S. and will expand to more countries, including Japan, upon approval.
Said Dr. Khaldoun Tarakji, VP and CMO of Medtronic’s Cardiac Ablation Solutions business: “During development, our team designed the system to enable future software enhancements in the field. … We will always be committed to innovation that meets physicians’ needs in order to provide the best care for patients globally.”
