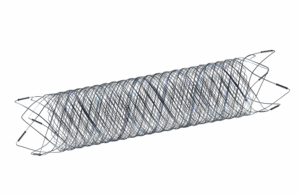
Terumo Neuro's MicroVention issued an urgent medical device recall of some Fred 27 and Fred X 27 Flow re-direction endoluminal devices. The company is voluntarily recalling certain lots of the devices due to complaints of a manufacturing error related to the tantalum length and attachment pattern. Terumo Neuro stated that the complaints have also … [Read more...] about Terumo Neuro recalls some Fred 27 neurovascular stents over tantalum wire issue
microvention
Terumo Neuro launches new stroke catheter in the U.S.
Terumo Neuro (formerly MicroVention) announced that it launched its Sofia 88 neurovascular support catheter in the U.S. Sofia 88 expands the company's stroke portfolio with a new large-bore catheter engineered for reliability, flexibility and physician control. The company says its launch builds upon the proven performance and legacy of the … [Read more...] about Terumo Neuro launches new stroke catheter in the U.S.
Terumo Neuro wins FDA nod for first dual-layer carotid stent
Terumo Neuro (formerly MicroVention) announced today that it received FDA premarket approval (PMA) for its Carotid Stent System. Aliso Viejo, California-based Terumo Neuro says this marks the first dual-layer micromesh carotid stent approved in the U.S. It offers physicians a clinically proven option to improve patient outcomes in carotid artery … [Read more...] about Terumo Neuro wins FDA nod for first dual-layer carotid stent
MicroVention has positive results in aneurysm occlusion device study
Terumo subsidiary MicroVention announced recent one-year study results supporting the use of its nitinol WEB 17 occlusion device. The company shared results from its multicenter, prospective CLEVER study evaluating WEB 17. It looked at the device in ruptured and unruptured aneurysms. The study aimed to understand the safety and effectiveness of … [Read more...] about MicroVention has positive results in aneurysm occlusion device study
MicroVention launches new stent in the U.S.
Terumo subsidiary MicroVention announced that it began the U.S. launch of its LVIS EVO intraluminal support device. The device is now available in the U.S. for the treatment of wide-neck intracranial aneurysms. Aliso Viejo, California-based MicroVention first launched the device in Europe in 2019, selling more than 12,000 units … [Read more...] about MicroVention launches new stent in the U.S.
MicroVention announces first U.S. clinical case using aneurysm flow diverter Fred X
MicroVention today announced that the first U.S. clinical case of its flow diverter Fred X device was performed at Thomas Jefferson University Hospital in Philadelphia. Aliso Viejo, California-based MicroVention received premarket approval for the Fred X device in September. It uses a self-expanding braided nitinol mesh to redirect blood flow … [Read more...] about MicroVention announces first U.S. clinical case using aneurysm flow diverter Fred X