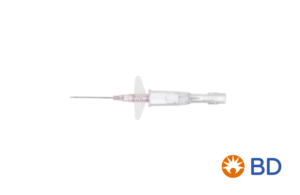
 BD: Cathena safety IV catheter with Multiguard technology
BD: Cathena safety IV catheter with Multiguard technology
BD (NYSE:BDX) noted its Cathena safety IV catheter with Multiguard Technology as one of its pipeline devices expected to launch 2021 in an investor presentation.
The Cathena safety IV catheter is ergonomically designed with the company’s Multiguard technology to provide a safe insertion to reduce the risk of blood exposure to a clinician and patient while also protecting them from accidental needlesticks.
The Multiguard technology delivers multi-use blood control and eliminates the need for venous compression during subsequent connections and disconnections to reduce exposure to blood-borne pathogens, according to the company. The catheter has a passive safety system that shields the needle as it’s withdrawn and its Instaflash needle technology provides immediate feedback of successful vessel entry to reduce the number of insertion attempts.
BD’s InstaFlash Needle Technology confirms immediate visual vessel entry, increasing clinician opportunities for first attempt insertion success, as supported by a recent clinical trial. The triple-flash system also provides two opportunities for confirmation in the flash chamber and in the catheter tub for more successful IV insertions.
Other devices BD had in its pipeline presentation include:
- Proxis 11/13.
- Global Intermittent Self Cath.
- Crosser IQ.
- Glidepath LE.

