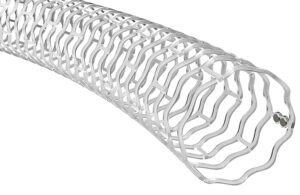
Abbott (NYSE: ABT) announced today that it received CE mark for its Esprit BTK everolimus-eluting resorbable scaffold system.
Esprit BTK offers treatment for people with peripheral artery disease (PAD) below the knee (BTK). Abbott designed it to keep arteries open and deliver the drug everolimus to support vessel healing. Following the therapy, the stent completely dissolves over time.
According to Abbott, Esprit BTK is the first resorbable scaffold of its kind for use BTK. The company constructed the stent from material similar to dissolving sutures. Esprit BTK is implanted through a minimally invasive, catheter-based procedure via a small incision in the leg.
Once the stent clears the blockage, the scaffold helps seal the vessel and provide support until the vessel can remain open on its own. After that point, the scaffold resorbs.
Esprit picked up FDA clearance in April 2024. Abbott last fall reported positive LIFE-BTK study outcomes for the system compared to balloon angioplasty. It can now begin marketing efforts for the stent in Europe with CE mark.
“Abbott is an expert in the development of innovative treatments for vascular diseases, and pioneered dissolving stents for people with PAD below the knee,” said Samih Al Mawass, divisional VP of Europe, Middle East & Africa (EMEA) at Abbott’s Vascular business. “With the Esprit BTK System, we’re helping to restore blood flow without leaving a permanent implant behind. Our resorbable program is focused on delivering meaningful innovation in the peripheral anatomy to help patients live healthier, fuller lives.”
