Micro-Tech Endoscopy USA announced that it launched its new ABC Balloon for esophageal, pyloric or colonic dilation. Ann Arbor, Mich.-based Micro-Tech's new balloon expands its current line of products, with six available diameter size, all with a balloon length of 5.5 cm, according to a news release. Micro-Tech said in the release that its … [Read more...] about Micro-Tech Endoscopy launches new dilation balloon
Boston Scientific CEO Mike Mahoney talks Lotus decision, post-COVID outlook
Boston Scientific (NYSE:BSX) made waves this month when it recalled and retired its Lotus TAVR product line. Subsequent layoffs, first in Minnesota, then in Ireland, highlighted the impact of the decision to pull Lotus from the highly competitive market as a result of complexities related to the product delivery system and solely that — as the … [Read more...] about Boston Scientific CEO Mike Mahoney talks Lotus decision, post-COVID outlook
CHF Solutions seeks patent for new Aquadex SmartFlow tech
CHF Solutions (NSDQ:CHFS) announced today that it submitted a patent application for a new advance to its Aquadex SmartFlow system. Eden Prairie, Minn.-based CHF Solutions develops Aquadex SmartFlow consoles for removing excess fluid from patients suffering from hypervolemia (fluid overload), which have recently been in demand due to … [Read more...] about CHF Solutions seeks patent for new Aquadex SmartFlow tech
Philips touts post-stent imaging study results
Royal Philips (NYSE:PHG) announced today that one-year results of its Define PCI study that reported improved outcomes and less recurrent angina. The Define PCI study assessed the level of residual ischemia found in patients following a percutaneous coronary intervention (PCI) using a blinded instant wave-free ratio (iFR) pullback measurement, … [Read more...] about Philips touts post-stent imaging study results
NanoVibronix device gains FDA nod for import to U.S.
NanoVibronix (NSDQ:NAOV) announced that it received FDA enforcement discretion for the U.S. distribution of the UroShield device. According to a news release, the FDA exercised its enforcement discretion, giving Elmsford, N.Y.-based NanoVibronix’s UroShield an intended use code (IUC), clearing the way for the import of the system to the U.S. … [Read more...] about NanoVibronix device gains FDA nod for import to U.S.
Boston Scientific warns on Hurricane RX biliary balloon dilatation catheter
Boston Scientific (NYSE:BSX) said it is recalling specific lots/batches of its Hurricane RX biliary balloon dilation catheters. Marlborough, Mass.-based Boston Scientific, which issued a warning letter from its international location in France, said the removal comes in response to an increase in complaints of the RX tunnel component (black … [Read more...] about Boston Scientific warns on Hurricane RX biliary balloon dilatation catheter
Saint-Gobain Life Sciences buys MS Techniques and Transluminal
Saint-Gobain Life Sciences announced today that it is acquiring MS Techniques and Transluminal to expand its medical components capabilities. Pompey, France-based MS Techniques, a 137-person company, offers experience in high-precision thermoplastic extrusion and design expertise for minimally invasive catheter solutions centered around the … [Read more...] about Saint-Gobain Life Sciences buys MS Techniques and Transluminal
Medtronic touts cryoablation therapy study results
Medtronic (NYSE:MDT) touted results demonstrating the superiority of two of its cryoablation therapy technologies. Fridley, Minn.-based Medtronic said in a news release that trials showed the superiority of the Arctic Front advance cardiac cryoballoon and the Freezor max cardiac cryoablation catheter for the first-line treatment (before drug … [Read more...] about Medtronic touts cryoablation therapy study results
AngioDynamics lands 510(k) for ‘smart’ implantable port
AngioDynamics (NSDQ:ANGO) announced that it received FDA 510(k) clearance and CE Mark approval for its SmartPort+ implantable port product. SmartPort+ is an implantable, subcutaneous port for those who require long-term access to the central venous system for blood specimen withdrawal and administration of fluids, according to a news … [Read more...] about AngioDynamics lands 510(k) for ‘smart’ implantable port
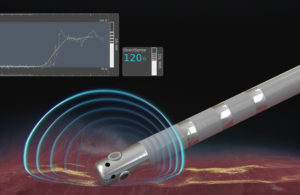
Boston Scientific launches DirectSense technology
Boston Scientific (NYSE:BSX) announced today that it launched its DirectSense for monitoring the effect of radiofrequency (RF) energy during cardiac ablation procedures. Marlborough, Mass.-based Boston Scientific’s DirectSense is available on the Rhythmia HDx mapping system that won FDA approval in April. Rhythmia HDx monitors changes in local … [Read more...] about Boston Scientific launches DirectSense technology