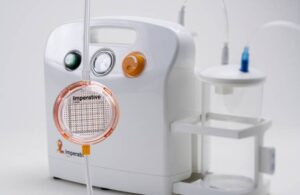
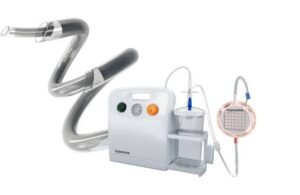
Imperative Care announced new real-world data evaluating ADAPT 2.0, a next-generation approach to stroke treatment. ADAPT (A Direct Aspiration First Pass Technique) 2.0 combines 0.088” intracranial access, asymmetric aspiration, and the continuous dual aspiration technique (CDAT) to provide fast and effective clot removal. Campbell, … [Read more...] about Imperative Care reports positive results for next-gen stroke aspiration approach
Neurology
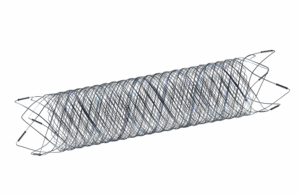
Terumo Neuro recalls some Fred 27 neurovascular stents over tantalum wire issue
Terumo Neuro's MicroVention issued an urgent medical device recall of some Fred 27 and Fred X 27 Flow re-direction endoluminal devices. The company is voluntarily recalling certain lots of the devices due to complaints of a manufacturing error related to the tantalum length and attachment pattern. Terumo Neuro stated that the complaints have also … [Read more...] about Terumo Neuro recalls some Fred 27 neurovascular stents over tantalum wire issue
Penumbra promotes from within to fill president position
Penumbra (NYSE:PEN) announced today that it promoted Shruthi Narayan to the position of president of the company. Narayan's appointment goes into effect on Sept. 1, 2025. She continues to report to Adam Elsesser, who previously held the post of president before this promotion. Elsesser continues on as the Penumbra's chair and CEO. The … [Read more...] about Penumbra promotes from within to fill president position
Autonomix brings nerve-sensing catheter closer to clinical studies
Autonomix Medical (Nasdaq:AMIX) announced today that it advanced its proprietary sensing catheter through a final design review. This marks the latest step for the Texas-based company in preparation for human clinical trials. Previous versions used in animal models demonstrated the ability to detect nerve signals. Now, the company believes it's … [Read more...] about Autonomix brings nerve-sensing catheter closer to clinical studies
Imperative Care wins FDA nod for new Zoom 7X catheter
Imperative Care announced today that it received FDA 510(k) clearance for its novel Zoom 7X catheter for aspiration thrombectomy procedures. The company also reported the first patient cases with its latest advancement for ischemic stroke treatment. Campbell, California-based Imperative Care said it marks the first catheter to incorporate its … [Read more...] about Imperative Care wins FDA nod for new Zoom 7X catheter
Imperative Care has positive stroke treatment study results
Imperative Care today announced study data evaluating aspiration with its Zoom system in stroke patients with M2 occlusions. Campbell, California–based Imperative Care designed Zoom for fast and effective clot removal from access through reperfusion. It treats patients with acute ischemic stroke, enabling smooth tracking through challenging … [Read more...] about Imperative Care has positive stroke treatment study results
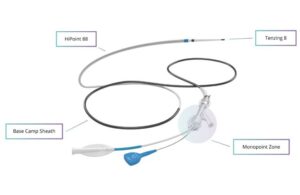
Real-world data backs Route 92 reperfusion system
Route 92 Medical today announced results from a real-world study evaluating reperfusion outcomes with its HiPoint system. San Mateo, California-based Route 92’s HiPoint system includes the HiPoint 88 system powered by Tenzing 8, featuring the Monopoint approach. The company says it became the first neurovascular intervention company to win … [Read more...] about Real-world data backs Route 92 reperfusion system
InspireMD launches carotid stent in U.S. after FDA approval
InspireMD (Nasdaq:NSPR) announced today that it launched its CGuard Prime carotid stent in the U.S. after receiving FDA premarket approval (PMA) last month. The CGuard embolic prevention stent (EPS) system — designed to prevent stroke — received CE mark last month as well. It utilizes the company’s MicroNet technology for strong acute results and … [Read more...] about InspireMD launches carotid stent in U.S. after FDA approval
FDA grants PMA for InspireMD CGuard Prime carotid stent, triggers $18M milestone payment
InspireMD announced that the FDA granted premarket approval (PMA) for its CGuard Prime carotid stent system. The CGuard embolic prevention stent (EPS) system — designed to prevent stroke — received CE mark earlier this month. It utilizes the company’s MicroNet technology for strong acute results and durable, stroke-free, long-term … [Read more...] about FDA grants PMA for InspireMD CGuard Prime carotid stent, triggers $18M milestone payment
Anaconda Biomed wins CE mark for funnel catheter
Anaconda Biomed announced today that it received CE mark approval to commercialize its ANA5 funnel catheter in Europe. The company designed ANA5, a next-generation funnel catheter, to optimize mechanical thrombectomy. It maximizes clot capture with its vessel-matching diameter funnel. Simultaneously, it enables antegrade flow arrest and offers the … [Read more...] about Anaconda Biomed wins CE mark for funnel catheter