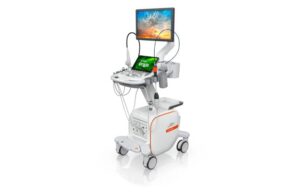
Acuson Origin, a new, dedicated cardiovascular ultrasound, includes AI-powered features. It can help physicians perform cardiac procedures more efficiently in the areas of diagnostics, structural heart disease and electrophysiology. The AcuNav Lumos 4D ICE (intracardiac echocardiography) catheter provides advanced imaging for complex heart procedures. It’s available exclusively with Acuson Origin.
Siemens Healthineers said in a news release that Acuson Origin addresses the entire continuum of cardiovascular patient care. It minimizes the cognitive and ergonomic workload of cardiac exams and procedures. This, in turn, allows for greater consistency, reproducibility and user efficiency.
The system’s suite of AI-powered features helps with measurements, view recognition and imaging. These features can save the user time on echocardiography procedures with accurate and reproducible automated measurements. Its capabilities include more than 5,600 AI-powered measurements on transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE).
Acuson Origin provides automated contouring and quantifiation of all four cardiac chambers without needing an ECG. The fourth-generation AcuNav Lumos catheter enhances procedural guidance for better patient care as well. It enables the treatment of patients otherwise unable to undergo structural heart interventions.
Features include improved MPR biplane imaging for more accurate anatomical assessment and guidance, plus improved leak detection.
“With its advanced AI features and potential to enhance diagnostic accuracy as well as patient care, the ACUSON Origin is positioned to reshape healthcare’s approach to cardiovascular imaging,” said David Zollinger, head of Cardiovascular Ultrasound at Siemens Healthineers. “And the AcuNav Lumos is the latest milestone in our 24-year history with ICE. Since 1999, our ICE catheters have helped more than 2 million patients, reducing the need for anesthesia during procedures.”
