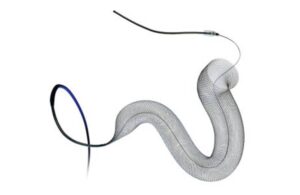
The recall involves removing Pipeline Vantage 027 device models from use and sale and updating instructions for Pipeline Vantage 021 models. Medtronic’s Pipeline Vantage embolization devices with Shield Technology treat bulges in the artery walls (aneurysms) of the brain. The endovascular devices go into blood vessels through a small catheter to the aneurysm location. There, a small, braided tube blocks off blood flow to the bulging area.
According to an FDA notice, Medtronic recalled and updated instructions for the devices due to reports of a higher incidence of the flexible, braided tube part of the device failing to attach or stay attached to the blood vessel wall (incomplete wall apposition and/or braid deformation) during and after procedures using the devices.
Incomplete wall apposition and braid deformation (also called fish-mouthing), braid narrowing or braid collapse could occur. These risks proved higher in females, especially those younger than 45 years old. Medtronic says using the affected product could cause serious health consequences, including thrombosis, stroke or death.
Medtronic reports 13 injuries and four deaths related to the Pipeline Vantage 027 device. It notes four reported injuries and no deaths with the Pipeline Vantage 021 device.
On Jan. 30, 2025, Medtronic sent an urgent medical device recall letter recommending that customers do not use and return all unused 027 devices. They should read the updated instructions before using the 021 devices. The instruction updates aim to help achieve optimal size selection and stent braid deployment.
For patients already treated with the affected devices, the treating physician should determine the need for follow-up imaging or changes to medical management based on the patient’s overall health. This includes weighing the risks of dual antiplatelet therapy against potential risks for braid deformation.
