The FDA issued a notice highlighting a letter issued by BD and its C.R. Bard Urology and Critical Care subsidiary warning of new balloon device instructions. This warning follows one death associated with the device issue. BD sent affected customers a notice updating instructions for all lots of certain esophagogastric balloon tamponade tubes. … [Read more...] about BD warns on esophagogastric balloon tamponade tubes after death
BD Bard
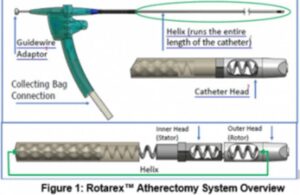
BD warns on atherectomy catheters after deaths
BD (NYSE: BDX) recently issued a medical device correction later related to its Bard subsidiary's Rotarex atherectomy system. The company identified a number of anatomical factors that could contribute to catheter helix fracture and/or breakage. It reports 30 serious injuries and four deaths associated with the issue. Additionally, BD reported … [Read more...] about BD warns on atherectomy catheters after deaths