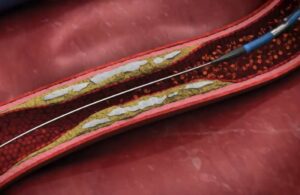
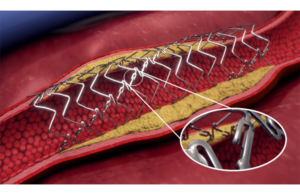
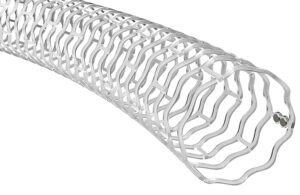
Elixir Medical announced today that it received CE mark for its LithiX hertz contact (HC) intravascular lithotripsy (IVL) system. The Milpitas, California–based company kicked off its commercial launch for the IVL system in Europe following receipt of CE mark. Dr. Stefano Galli (Centro Cardiologico Monzino in Milan), Dr. Eric Van Belle (Lille … [Read more...] about Elixir Medical launches IVL system in Europe
Elixir Medical
Elixir Medical has positive data for drug-eluting bioadaptive implant
Elixir Medical announced positive data demonstrating the benefit of its DynamX coronary bioadaptor system in target lesion failure (TLF). Data highlighted the success of DynamX compared to Medtronic's Resolute Onyx zotarolimus drug-eluting stent (DES). The study looked at complex patient populations within the INFINITY-SWEDEHEART randomized … [Read more...] about Elixir Medical has positive data for drug-eluting bioadaptive implant
Bioabsorbable polymers for implantable medical devices: What to know
Bioabsorbable polymers are used for an expanding range of medical devices.
Bioabsorbable polymers degrade and disappear at predictable rates, making them an ideal material for parts of implantable devices that could otherwise impair healing or create an ongoing risk of injury or infection.
Bioabsorbable sutures made of glycolide/lactide polymers, first developed in the 1970s, are strong and flexible enough to hold … [Read more...] about Bioabsorbable polymers for implantable medical devices: What to know