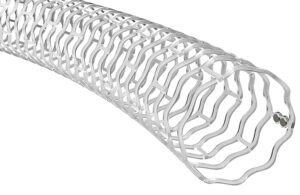
Boston Scientific announced recently that it received CE mark for its Farapoint pulsed field ablation (PFA) catheter. The regulatory nod, earned last week, allows the Marlborough, Massachusetts-based company to bring the newest PFA catheter in its electrophysiology portfolio to the European market. Farapoint treats right atrial flutter (AFL), … [Read more...] about Boston Scientific gets European nod for Farapoint catheter
CE Mark
AngioSafe emerges from stealth with FDA clearance for Santreva-ATK catheter
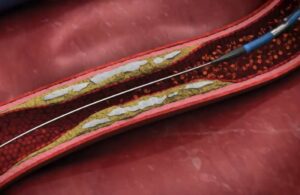
AngioSafe announced it has received FDA 510(k) clearance and CE marking for its Santreva-ATK endovascular revascularization catheter. San Jose, California–based AngioSafe designed the device for wire-free intraluminal crossing of chronic total occlusions (CTOs) while simultaneously compressing plaque and recanalizing vessels. Santreva-ATK enables … [Read more...] about AngioSafe emerges from stealth with FDA clearance for Santreva-ATK catheter
MicroPort Cardio’s Alwide Plus balloon catheter wins CE mark
MicroPort CardioFlow said its Alwide Plus balloon catheter has received CE mark approval, clearing the company to market the device in Europe. The approval makes Alwide Plus the company’s fourth product authorized in Europe, following the VitaFlow Liberty transcatheter aortic valve and delivery system, and the AnchorMan left atrial appendage … [Read more...] about MicroPort Cardio’s Alwide Plus balloon catheter wins CE mark
Abbott wins CE mark for Esprit drug-eluting, dissolving scaffold
Abbott (NYSE: ABT) announced today that it received CE mark for its Esprit BTK everolimus-eluting resorbable scaffold system. Esprit BTK offers treatment for people with peripheral artery disease (PAD) below the knee (BTK). Abbott designed it to keep arteries open and deliver the drug everolimus to support vessel healing. Following the therapy, … [Read more...] about Abbott wins CE mark for Esprit drug-eluting, dissolving scaffold
Anaconda Biomed wins CE mark for funnel catheter
Anaconda Biomed announced today that it received CE mark approval to commercialize its ANA5 funnel catheter in Europe. The company designed ANA5, a next-generation funnel catheter, to optimize mechanical thrombectomy. It maximizes clot capture with its vessel-matching diameter funnel. Simultaneously, it enables antegrade flow arrest and offers the … [Read more...] about Anaconda Biomed wins CE mark for funnel catheter
InspireMD wins CE Mark approval for CGuard Prime
InspireMD today announced it received CE Mark approval for its CGuard Prime carotid stent system for the prevention of stroke. The Miami-based company said the next-generation carotid stent system was developed based on physician feedback and features improved deliverability and deployment. It builds on the original CGuard design, which uses a … [Read more...] about InspireMD wins CE Mark approval for CGuard Prime
Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Medtronic announced today that it received CE mark for several expanded indications for its Prevail balloon catheter. The expanded indications for the paclitaxel-coated percutaneous transluminal coronary angioplasty (PTCA) balloon catheter (or drug-coated balloon/DCB) cover the treatment of coronary artery disease (CAD). According to the medtech … [Read more...] about Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Elixir Medical launches IVL system in Europe
Elixir Medical announced today that it received CE mark for its LithiX hertz contact (HC) intravascular lithotripsy (IVL) system. The Milpitas, California–based company kicked off its commercial launch for the IVL system in Europe following receipt of CE mark. Dr. Stefano Galli (Centro Cardiologico Monzino in Milan), Dr. Eric Van Belle (Lille … [Read more...] about Elixir Medical launches IVL system in Europe
Boston Scientific wins CE mark for Farapulse Nav mapping tech
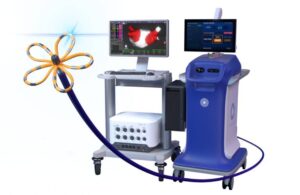
Boston Scientific announced today that it received CE mark for its navigation-enabled Farawave Nav ablation catheter and Faraview software. Farawave Nav and the Faraview software make up part of the company’s Farapulse pulsed field ablation (PFA) system. Boston Scientific initially won FDA approval for Farapulse in January 2024. In doing so, it … [Read more...] about Boston Scientific wins CE mark for Farapulse Nav mapping tech
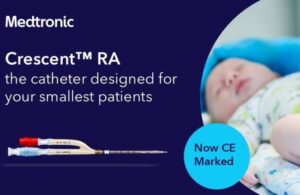
Medtronic wins CE mark for jugular dual lumen catheter for young children
A Medtronic official said today on LinkedIn that the company won CE mark for its Crescent RA jugular dual lumen catheter. Giuseppe Savoja, the medtech giant's senior business director for Western Europe, posted the announcement on LinkedIn: "Exciting times! I'm proud and thrilled to announce that the Medtronic Crescent RA jugular dual lumen … [Read more...] about Medtronic wins CE mark for jugular dual lumen catheter for young children