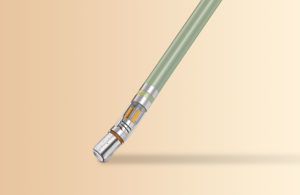
6. Johnson & Johnson: Thermocool SmartTouch SF ablation catheter
Johnson & Johnson’s Biosense Webster business won FDA approval for its Thermocool SmartTouch SF ablation catheter for treatment of persistent atrial fibrillation in October 2020.
The ThermoCool SmartTouch catheter has a porous tip that provides uniform cooling at half the flow rate of earlier generations of irrigated catheters, according to Biosense Webster. The catheter is integrated with the company’s Carto 3 system, which combines contact force technology, 3D mapping and advanced navigation capabilities to provide active measurement of stable contact force and catheter tip location.
The unidirectional catheter also provides consistent movement in response to contact force and sends accurate location reference signals through a location sensor and transmitter coil, according to J&J. The catheter helps promote consistent lesion formation, reduces ablation time and improves outcomes with more stable catheter-tissue contact force, the company said.

