Freudenberg Medical recently announced that it has increased its catheter sensor integration capabilities. The expanded capabilities allow the company to work with sensors for temperature and pressure, entire sensor arrays and fiber optic sensors in catheter manufacturing. "At Freudenberg Medical, we understand the difficulty in handling … [Read more...] about Freudenberg Medical touts expanded catheter sensor integration capabilities
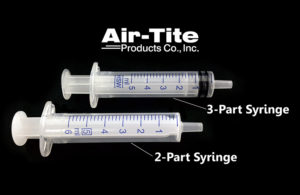
What is the difference between two-part and three-part syringes?
Syringes remain an integral part of the medical industry, yet they are often an afterthought in the R&D or production phase. William Foley, Air-Tite Products [Image courtesy of Air-Tite Products] With the wide variety of options available on the market containing fundamental differences in design and composition, can the syringe selection … [Read more...] about What is the difference between two-part and three-part syringes?
FDA clears Boston Scientific’s Exalt Model B single-use bronchoscope
Boston Scientific (NYSE:BSX) announced today that it received FDA 510(k) clearance for its Exalt Model B single-use bronchoscope. Marlborough, Mass.-based Boston Scientific designed the Exalt Model B for use in bedside procedures within the intensive care unit and operating room, according to a news release. Uses for the device include a wide … [Read more...] about FDA clears Boston Scientific’s Exalt Model B single-use bronchoscope
FDA clears 3NT Medical single-use sinus endoscope
3NT Medical today announced its Peregrine Drivable ENT scope has received FDA 510(k) clearance. Short Hills, N.J.-based 3NT Medical designed the Peregrine as a single-use endoscope that gives ENT surgeons views of the sinus anatomy. It gives surgeons access to the furthest reaches of the sinus to visualize anatomic landmarks up close with … [Read more...] about FDA clears 3NT Medical single-use sinus endoscope
Penumbra sales grow 75% in Street-beating Q2 results
Penumbra (NYSE:PEN) posted second-quarter results this week that beat the overall consensus on Wall Street. The Alameda, Calif.-based vascular conditions device company reported profits of $9.2 million, or 25¢ per share, on sales of $184.3 million for the three months ended June 30, for a sales growth of 75.3% compared with Q2 2020. Adjusted … [Read more...] about Penumbra sales grow 75% in Street-beating Q2 results
Intersect ENT launches VenSure balloon sinus dilation system in U.S.
Intersect ENT today announced that it has commercially launched its VenSure balloon sinus dilation system and Cube 4D navigation system in the U.S. Menlo Park, Calif.-based Intersect ENT designed the VenSure balloon and Cube 4D navigation system to be used in procedures that improve debilitating chronic rhinosinusitis (CRS) symptoms. Cube 4D has … [Read more...] about Intersect ENT launches VenSure balloon sinus dilation system in U.S.
Medtronic launches Prevail drug-coated balloon catheter in Europe
Medtronic today announced that it has launched its Prevail drug-coated balloon catheter in Europe. Dublin-based Medtronic designed the Prevail catheter for percutaneous coronary intervention procedures to treat narrowed or blocked coronary arteries in patients with coronary artery disease. During the procedure, the balloon inflates within the … [Read more...] about Medtronic launches Prevail drug-coated balloon catheter in Europe
The 18 most innovative medical devices of 2021
The Galien Foundation today announced the nominees for most innovative medical devices for its 15th annual Prix Galien USA Awards. The foundation nominates devices, biotechnology and pharmaceutical products for its annual Prix Galien awards to highlight products designed to improve the human condition. “The Awards Committee is excited to … [Read more...] about The 18 most innovative medical devices of 2021
VistaMed, OneProjects awarded €5M grant for cardiac arrhythmia catheter development
Freudenberg Medical's VistaMed today said it is a part of a team that was awarded €5.1 million ($6 million) over a three-year period to develop a catheter to improve treatment of cardiac arrhythmia. VistaMed, along with OneProjects and the Tyndall Institute, received the grant sponsored by the Irish government through the Disruptive Technology … [Read more...] about VistaMed, OneProjects awarded €5M grant for cardiac arrhythmia catheter development
FDA clears Stryker biodegradable subacromial balloon spacer
Stryker (NYSE:SYK) this week announced that it received FDA de novo clearance for its balloon implant for arthroscopic treatment of massive irreparable rotator cuff tears. Kalamazoo, Mich.–based Stryker designed the InSpace balloon implant to restore the subacromial space without requiring sutures or fixation devices. The device has demonstrated … [Read more...] about FDA clears Stryker biodegradable subacromial balloon spacer