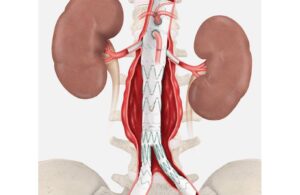
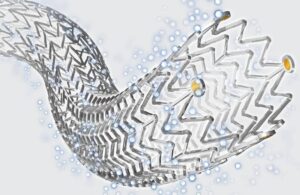
Cook Medical announced that it enrolled the final patient in the global clinical study of its Zenith Fenestrated+ Endovascular Graft (ZFEN+). ZFEN+ won FDA investigational device exemption (IDE) in June 2023. This study, conducted under that IDE, assesses ZFEN+'s safety and effectiveness used in combination with the investigational Zenith … [Read more...] about Cook treats final patient in endovascular graft study
Cook Medical
Cook Medical angiographic catheter recall is Class I
The FDA has designated Cook Medical's recall of its Beacon Tip 5.0 Fr angiographic catheter as Class I, the most serious level. Users reported tip separation occurring both before and during patient contact, according to an FDA notice published today. As a result, catheter fragmentation and embolization might occur with the catheters involved in … [Read more...] about Cook Medical angiographic catheter recall is Class I
Cook Medical warns of issue with angiographic catheter
Cook Medical issued an urgent field safety notice in Europe to warn of an issue with its Beacon Tip 5.0 Fr angiographic catheter. The company identified an issue in which affected device lots may experience tip separation. This prompted Cook to request that customers remove devices from use, quarantining them and returning them to the … [Read more...] about Cook Medical warns of issue with angiographic catheter
Merit Medical completes acquisition of Cook Medical’s lead management portfolio
Merit Medical Systems (Nasdaq:MMSI) announced today that it completed its acquisition of Cook Medical’s lead management portfolio. South Jordan, Utah-based Merit announced its agreement to buy the portfolio for $210 million in September. Merit funded the acquisition through a combination of cash on hand and borrowings under a long-term credit … [Read more...] about Merit Medical completes acquisition of Cook Medical’s lead management portfolio
Cook Medical launches IVF device in U.S.
Cook Medical announced today that it launched its NestVT vitrification device for in-vitro fertilization (IVF) in the U.S. Bloomington, Indiana-based Cook designed NestVT as a secure solution for embryos and oocytes during vitrification, cryo-storage and relocation. The company says the launch coincides with an effort to become a trusted IVF … [Read more...] about Cook Medical launches IVF device in U.S.
Merit Medical to buy Cook Medical’s lead management portfolio
Merit Medical Systems (Nasdaq:MMSI) announced today that it agreed to acquire Cook Medical's lead management portfolio for $210 million. Cook Medical's lead management business provides medical devices and accessories used in lead management procedures. These procedures occur in patients who need a pacemaker or an implantable … [Read more...] about Merit Medical to buy Cook Medical’s lead management portfolio
Cook Medical wins DOD contract for drug-eluting stents
Cook Medical announced today that the U.S. Dept. of Defense (DOD) awarded it an ECAT contract for implantable medical devices. The contract includes implantable vascular medical devices like the Zilver PTX drug-eluting peripheral stent. It also applies to the Zenith aortic endografts and associated interventional devices for treating vascular … [Read more...] about Cook Medical wins DOD contract for drug-eluting stents
Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Getinge and Cook Medical today announced they signed a commercial distribution agreement for the iCast covered stent system in the U.S. In this deal, Cook Medical will take over sales, marketing, and distribution rights for the product in the U.S. The iCast covered stent system will continue to be manufactured by Atrium Medical, a Getinge … [Read more...] about Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Cook Medical signs distribution agreement with Bentley for BeBack catheter
Cook Medical today announced it signed a U.S. distribution agreement with Bentley for the BeBack catheter. Under the agreement, Cook Medical will assume commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack catheter to our peripheral intervention portfolio,” Alec Cerchiari, director of product … [Read more...] about Cook Medical signs distribution agreement with Bentley for BeBack catheter
Cook Medical’s shorter Liver Access and Biopsy Set wins expanded indication
Cook Medical this week announced its shorter Liver Access and Biopsy Set (LABS) received pediatric indication. LABS was originally intended for use in adults, but was recently FDA cleared for use in adolescents, children and infants. The Bloomington, Indiana-based company designed the device for use in obtaining liver histology samples via … [Read more...] about Cook Medical’s shorter Liver Access and Biopsy Set wins expanded indication