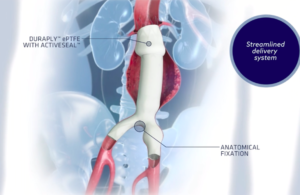
Abiomed (NSDQ:ABMD) announced today that the FDA granted premarket approval (PMA) to its Impella RP with SmartAssist. Danvers, Mass.–based Abiomed received PMA for the Impella RP right heart pump with SmartAssist as a safe and effective treatment for acute right heart failure for up to 14 days, according to a news release. Get the full story at … [Read more...] about FDA grants PMA to Abiomed’s next-generation Impella RP
fda
Medtronic wins expanded FDA approval for cryoablation catheters
Medtronic (NYSE:MDT) announced today that it received FDA expanded approval for its Arctic Front family of cardiac cryoablation catheters. Fridley, Minn.-based Medtronic's Arctic Front family of catheters treat recurrent symptomatic paroxysmal atrial fibrillation (AFib) as an alternative to antiarrhythmic drug (AAD) therapy as an initial rhythm … [Read more...] about Medtronic wins expanded FDA approval for cryoablation catheters
Medtronic wins FDA breakthrough nod for Emprint ablation catheter kit
Medtronic (NYSE:MDT) announced today that it received FDA breakthrough device designation for its Emprint ablation catheter kit. Fridley, Minn.-based Medtronic's catheter is designed to be used in conjunction with the Emprint microwave generator and the Medtronic lung navigation platform to offer a minimally invasive, localized treatment of … [Read more...] about Medtronic wins FDA breakthrough nod for Emprint ablation catheter kit
FDA says Cordis carotid artery stent recall is serious
The FDA has classified Cordis‘ recall of its Precise Pro Rx carotid system as Class I, the most serious kind of recall. Cordis in February recalled certain lots of its Precise Pro RX carotid stent systems because of a risk of separation of the atraumatic distal tip of the sheathed delivery system in patients. Get the full story at our sister … [Read more...] about FDA says Cordis carotid artery stent recall is serious
FDA clears Acutus Medical suite of universal transseptal crossing devices
Acutus Medical announced that it received FDA clearance for its AcQCross family of universal transseptal crossing devices. Carlsbad, Calif.-based Acutus touts the AcQCross system as the first and only transseptal puncture system specifically engineered to pair and mate seamlessly with Acutus' suite of sheaths, as well as with sheaths sold by … [Read more...] about FDA clears Acutus Medical suite of universal transseptal crossing devices
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
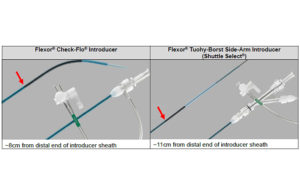
Cook Medical has a serious catheter recall
FDA has designated Cook Medical's recall of its Flexor Check-Flo introducers and Flexor tuohy-borst side-arm introducers as Class I, its most serious level. Cook Medical initiated the recall on Nov. 24. It involves 37,326 of the devices, which are used to deliver medical devices to blood vessels (though not vessels of the heart and brain). The … [Read more...] about Cook Medical has a serious catheter recall
SoniVie wins FDA breakthrough nod for renal denervation tech
SoniVie announced that it received FDA breakthrough device designation for its Tivus renal artery denervation technology. Tivus (therapeutic intra-vascular ultrasound system) is designed for treating resistant hypertension through renal artery denervation. The system previously received breakthrough designation for treating pulmonary arterial … [Read more...] about SoniVie wins FDA breakthrough nod for renal denervation tech
NanoVibronix device gains FDA nod for import to U.S.
NanoVibronix (NSDQ:NAOV) announced that it received FDA enforcement discretion for the U.S. distribution of the UroShield device. According to a news release, the FDA exercised its enforcement discretion, giving Elmsford, N.Y.-based NanoVibronix’s UroShield an intended use code (IUC), clearing the way for the import of the system to the U.S. … [Read more...] about NanoVibronix device gains FDA nod for import to U.S.
FDA labels Applied Medical catheter recall as Class I
The FDA announced yesterday that the Applied Medical recall of multiple kinds of its catheters is designated as Class I, the most serious type of recall. The Python embolectomy catheters, Bard embolectomy catheters and the OTW Latis cleaning catheters were the types recalled by Applied Medical. A total of 19,400 devices were recalled in the … [Read more...] about FDA labels Applied Medical catheter recall as Class I