Medtronic (NYSE: MDT) is recalling specific lots of its Shiley Adult Flexible Tracheostomy Tube with TaperGuard Cuff and reusable inner cannula due to an issue that could pose serious health risks. The FDA labeled the recall as Class I, the most serious kind, because the device’s securement flange may detach from the cannula, potentially … [Read more...] about Medtronic has a serious tracheostomy tube recall
medtronic
Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Medtronic announced today that it received CE mark for several expanded indications for its Prevail balloon catheter. The expanded indications for the paclitaxel-coated percutaneous transluminal coronary angioplasty (PTCA) balloon catheter (or drug-coated balloon/DCB) cover the treatment of coronary artery disease (CAD). According to the medtech … [Read more...] about Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Medtronic reports more positive outcomes with Affera PFA tech
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced positive clinical outcomes from two studies of its pulsed field ablation (PFA) technologies. The studies looked at the Affera family of technologies. Those include the next-generation Sphere-360 single-shot PFA catheter and the Sphere-9 combination mapping and … [Read more...] about Medtronic reports more positive outcomes with Affera PFA tech
Medtronic wins FDA nod for world’s smallest defibrillation lead
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] announced that it received FDA approval for its OmniaSecure defibrillation lead for right ventricle placement. It built OmniaSecure on its reliable SelectSecure Model 3830 pacing lead for catheter delivery. It adds to the wide-ranging Medtronic portfolio of lead solutions for precise … [Read more...] about Medtronic wins FDA nod for world’s smallest defibrillation lead
Boston Scientific Farapulse PFA system shows superiority in head-to-head study with Medtronic cryoablation system
Boston Scientific announced new study findings demonstrating the superiority of its Farapulse pulsed field ablation (PFA) system. Data published in the New England Journal of Medicine showed Farapulse's superiority in reducing atrial arrhythmia (AA) recurrence or treating symptomatic, drug-refractory paroxysmal atrial fibrillation (PAF) compared … [Read more...] about Boston Scientific Farapulse PFA system shows superiority in head-to-head study with Medtronic cryoablation system
Medtronic recalls embolization device after reported deaths
The FDA deemed a recall of some Medtronic Pipeline Vantage embolization devices serious after multiple deaths related to the device. The recall involves removing Pipeline Vantage 027 device models from use and sale and updating instructions for Pipeline Vantage 021 models. Medtronic's Pipeline Vantage embolization devices with Shield Technology … [Read more...] about Medtronic recalls embolization device after reported deaths
Medtronic enrolls first patient in drug-coated balloon study
Medtronic (NYSE: MDT) announced today that investigators enrolled the first patient in a pivotal trial of its Prevail drug-coated balloon (DCB). The study evaluates the paclitaxel-coated balloon catheter for in-stent restenosis (ISR) and de novo small vessel disease. Dr. Ziad Ali, director of the DeMatteis Cardiovascular Institute at St Francis … [Read more...] about Medtronic enrolls first patient in drug-coated balloon study
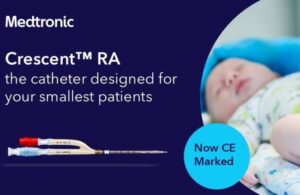
Medtronic wins CE mark for jugular dual lumen catheter for young children
A Medtronic official said today on LinkedIn that the company won CE mark for its Crescent RA jugular dual lumen catheter. Giuseppe Savoja, the medtech giant's senior business director for Western Europe, posted the announcement on LinkedIn: "Exciting times! I'm proud and thrilled to announce that the Medtronic Crescent RA jugular dual lumen … [Read more...] about Medtronic wins CE mark for jugular dual lumen catheter for young children
Medtronic inks stent distro deal with Contego with option to buy
Medtronic (NYSE: MDT)announced today that it entered into an exclusive U.S. distribution agreement with Contego Medical. Under the agreement, Medtronic serves as the sole U.S. distributor for Contego's portfolio of commercially available products. Raleigh, North Carolina-based Contego offers products that provide revascularization treatment for … [Read more...] about Medtronic inks stent distro deal with Contego with option to buy
Medtronic gets CE mark for Harmony transcatheter pulmonary valve
Medtronic today announced CE mark approval for its Harmony transcatheter pulmonary valve (TPV) system. About 1.3 children around the world each year are born with congenital heart disease, according to the Minneapolis-based nonprofit Children's HeartLink. The Harmony system provides a minimally invasive alternative to open-heart surgery for … [Read more...] about Medtronic gets CE mark for Harmony transcatheter pulmonary valve