A hospital in Texas announced today that its electrophysiologists completed the first U.S. cases with the Medtronic (NYSE:MDT) Affera platform. EPs at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David's Medical Center performed the procedures with Affera. Dr. Andrea Natale and Dr. Amin Al-Ahmad conducted the first procedure on Nov. 7. A … [Read more...] about First U.S. cases performed with Medtronic Affera ablation system
medtronic
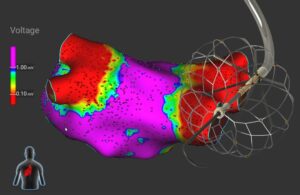
Medtronic Evolut Pro TAVR demonstrates positive outcomes in study
Medtronic (NYSE:MDT) this week shared new clinical data supporting its Evolut Pro/Pro+ transcatheter aortic valve replacement (TAVR) system. Findings included cusp overlap technique (COT) outcomes from the Optimize PRO clinical study and pooled analysis looking at Evolut Pro patients showing improvement in paravalvular leak (PVL) over time … [Read more...] about Medtronic Evolut Pro TAVR demonstrates positive outcomes in study
Medtronic acquires articulating instrument maker Fortimedix Surgical
Fortimedix Surgical announced today that it has been acquired by medtech giant Medtronic (NYSE:MDT). "We are pleased to share that Fortimedix Surgical was acquired by Medtronic," the company wrote on LinkedIn. "A unique opportunity to leverage our articulating instrument technology for application across patient therapies. We look forward to … [Read more...] about Medtronic acquires articulating instrument maker Fortimedix Surgical
FDA approves IDE for Medtronic Prevail drug-coated balloon
Medtronic (NYSE: MDT) announced today that it received FDA investigational device exemption (IDE) for its Prevail drug-coated balloon (DCB). The medtech giant can now begin a pivotal clinical trial for the coronary paclitaxel DCB for in-stent restenosis (ISR) and de novo small vessel disease. It plans to use data from its Prevail Global Clinical … [Read more...] about FDA approves IDE for Medtronic Prevail drug-coated balloon
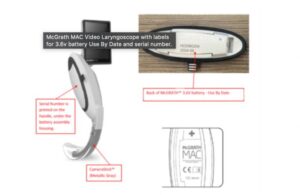
Medtronic recalls McGrath Mac laryngoscope due to potential for explosions
The FDA issued a notice deeming a recall of Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] McGrath Mac video laryngoscopes Class I, the most serious kind. This recall involves removing certain devices from where they are used or sold as the device may cause serious injury or death if people continue using it. It also includes … [Read more...] about Medtronic recalls McGrath Mac laryngoscope due to potential for explosions
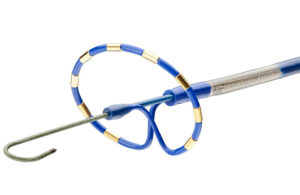
Pulsed field ablation catheters take shape at Medtronic, Boston Scientific, Abbott and J&J’s Biosense Webster
Experts from Medtech Big 100 device developers — Medtronic, Johnson & Johnson MedTech, Abbott and Boston Scientific — discuss the various shapes of their pulsed field ablation (PFA) catheters. From flowers and loops to globes, baskets and balloons, there's no standard shape for the pulsed field ablation (PFA) catheters coming out of Medtronic, … [Read more...] about Pulsed field ablation catheters take shape at Medtronic, Boston Scientific, Abbott and J&J’s Biosense Webster
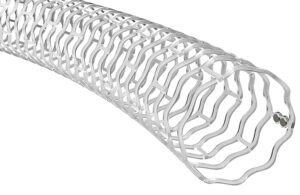
How Medtronic designed the PulseSelect pulsed field ablation system for AFib
Medtronic‘s PulseSelect became the first pulsed field ablation (PFA) system to win FDA approval for treating atrial fibrillation (AFib) in December 2023. The world’s largest medical device manufacturer achieved that milestone about 15 years after one of its engineers, Mark Stewart, coined the term “pulsed field ablation” in a research paper … [Read more...] about How Medtronic designed the PulseSelect pulsed field ablation system for AFib
Medtronic endotracheal tubes recall sparks an FDA warning
The FDA issued a notice instructing healthcare providers to stop using certain reinforced endotracheal tubes made by Medtronic (NYSE:MDT). This warning extends to NIM Contact EMG reinforced endotracheal tubes and NIM (Standard) EMG reinforced endotracheal tubes. Medtronic offers the neural integrity monitor (NIM) electromyogram (EMG) tubes for … [Read more...] about Medtronic endotracheal tubes recall sparks an FDA warning
Medtronic launches Steerant aortic guidewire
Medtronic (NYSE:MDT) today announced the launch of its Steerant aortic guidewire in the U.S. and Canada following the first patient cases. The medtech giant tailored Steerant to facilitate catheter placement and exchange during diagnostic or interventional aortic procedures. It designed the guidewire to support endovascular aneurysm repair … [Read more...] about Medtronic launches Steerant aortic guidewire
Bioabsorbable polymers for implantable medical devices: What to know
Bioabsorbable polymers are used for an expanding range of medical devices.
Bioabsorbable polymers degrade and disappear at predictable rates, making them an ideal material for parts of implantable devices that could otherwise impair healing or create an ongoing risk of injury or infection.
Bioabsorbable sutures made of glycolide/lactide polymers, first developed in the 1970s, are strong and flexible enough to hold … [Read more...] about Bioabsorbable polymers for implantable medical devices: What to know