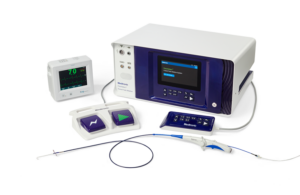
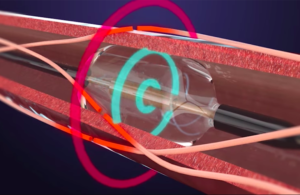
Medtronic said today that it's received a CE mark for its PulseSelect pulsed field ablation system. In addition, the medtech giant received European Union approval for its Nitron CryoConsole, which builds upon the legacy of the company's cryo franchise with features to optimize the workflow for cryoballoon ablation. The news of the regulatory … [Read more...] about Medtronic pulsed-field ablation system wins CE mark
Olympus has more warnings over bronchoscopes
Olympus has initiated a voluntary field corrective action following reports of endobronchial combustion incidents involving bronchoscopes. The field corrective action — announced Nov. 9 — is taking place after incidents occurred during medical procedures utilizing high-frequency therapy equipment in environments rich in oxygen, according to the … [Read more...] about Olympus has more warnings over bronchoscopes
Cordis has a new CEO
About two months after former Spectranetics CEO Scott Drake became executive board chair at Cordis, he's moved into the corner office at the cardiology and endovascular devices company. Drake replaces Shar Matin, who has stepped down as CEO, the company announced yesterday. Hunter Philbrick, Hellman & Friedman partner and company board … [Read more...] about Cordis has a new CEO
Access Vascular raises $22 million for production expansion
Access Vascular announced today that it has successfully raised $22 million in a Series C financing round. The funding round saw participation from both new and returning investors. Billerica, Massachusetts–based AVI said the funds will significantly boost production capacity for its Mimix hydrophilic biomaterial vascular access devices. … [Read more...] about Access Vascular raises $22 million for production expansion
B. Braun launches Introcan Safety 2 IV catheter
B. Braun Medical — known for its focus on infusion therapy — has released its Introcan Safety 2 IV catheter, equipped with multi-access blood control. This addition to B. Braun's line of passive needlestick prevention catheters emphasizes minimizing potential risks during the IV process. In addition to automatic needlestick protection, … [Read more...] about B. Braun launches Introcan Safety 2 IV catheter
Cordis names former Spectranetics CEO as executive chair
Interventional vascular tech company Cordis today announced that board member Scott Drake will be the new executive chair of its board of directors. Drake succeeds Duke Rohlen, who served as executive chair since Hellman & Friedman and KKR acquired the company from Cardinal Health in 2021. Rohlen will stay on the company's board of directors … [Read more...] about Cordis names former Spectranetics CEO as executive chair
ReCor Medical wins FDA panel nod for its renal denervation system
ReCor Medical announced that an FDA panel has voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. On Aug. 22, the Circulatory Systems Devices Panel of the FDA's Medical Devices Advisory Committee voted 12–0 in favor with regard to safety and 8–3 in favor, with one vote … [Read more...] about ReCor Medical wins FDA panel nod for its renal denervation system
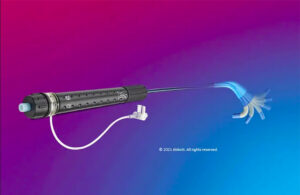
Abbott has a serious recall involving its Amplatzer delivery sheath
The FDA today said an Abbott recall involving its Amplatzer steerable delivery sheath is Class I, its most serious level. Launched in the U.S. in April 2022, the Amplatzer sheath delivers Abbott's Amplatzer Amulet left atrial appendage occluder. Competing against Boston Scientific's Watchman, Amplatzer Amulet provides complete occlusion in order … [Read more...] about Abbott has a serious recall involving its Amplatzer delivery sheath
Olympus corrective action covers laser-compatible bronchoscopes
Olympus is warning health providers about incompatible laser use with bronchoscopes after receiving reports of endobronchial combustion, serious patient injury, and one death. The Japanese medtech giant said on July 3 that its voluntary field corrective action involves 32 BF series endoscope models, 19 of which it distributes in the U.S. The … [Read more...] about Olympus corrective action covers laser-compatible bronchoscopes
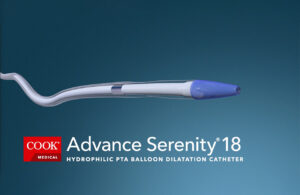
Cook Medical has more hydrophilic PTA balloon catheter options
Cook Medical today announced more sizes and locations for the Advance Serenity hydrophilic PTA balloon catheter product line. It's now possible for U.S. and Canadian interventionalists to use Advance Serenity for below-the-knee and above-the-knee procedures to treat patients with peripheral artery disease (PAD), according to Cook Medical. In … [Read more...] about Cook Medical has more hydrophilic PTA balloon catheter options