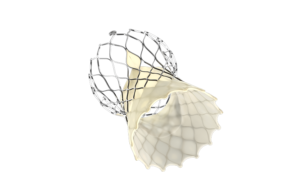
Medtronic today announced that its Evolut FX TAVR system has won FDA approval. Fridley, Minn.–based Medtronic designed the self-expanding transcatheter aortic valve replacement system to treat symptomatic severe aortic stenosis. The TAVR system uses a supra-annular valve design that has demonstrated hemodynamic performance that is superior to … [Read more...] about FDA approves Medtronic next-gen Evolut FX TAVR system
Nordson to acquire NDC Technologies
Nordson Corporation today announced that it has signed a definitive agreement to acquire Spectris's NDC Technologies for $180 million. Dayton, Ohio-based NDC Technologies makes in-line measurement sensors, gauges and analyzers using near-infrared, laser, X-ray, optical and nucleonic technologies. The acquisition will expand Westlake, Ohio-based … [Read more...] about Nordson to acquire NDC Technologies
Q’Apel Medical launches Armadillo radial access catheter
Q'Apel Medical today announced that its Armadillo radial access system won FDA 510(k) clearance. Fremont, Calif.-based Q'Apel Medical designed the Armadillo to help physicians move from femoral artery access to radial artery access. The company's radial platform of access catheters is specifically designed to address challenges associated with … [Read more...] about Q’Apel Medical launches Armadillo radial access catheter
Argon Medical Devices launches Skater Mini-Loop drainage catheter
Argon Medical Devices today announced that it has commercially launched its Skater Mini-Loop drainage catheter in the U.S. and Europe. Frisco, Texas-based Argon developed the catheter as a minimally invasive way to remove or drain unwanted fluid collection. It is an expansion of the company's Skater All-Purpose and Nephrostomy drainage … [Read more...] about Argon Medical Devices launches Skater Mini-Loop drainage catheter
Sinomed launches HT Supreme drug-eluting stent in Europe
Sinomed has completed the first commercial implantation of its HT Supreme drug-eluting stent in Ireland. Faisal Sharif, professor of translational cardiovascular medicine and innovation at the National University of Ireland Galway, successfully performed the first procedure with HT Supreme, a healing-targeted drug-eluting stent designed to treat … [Read more...] about Sinomed launches HT Supreme drug-eluting stent in Europe
FDA grants breakthrough device designation for Abiomed’s Impella ECP heart pump
Abiomed (NSDQ:ABMD) this week announced that the FDA has granted breakthrough device designation for its Impella ECP expandable percutaneous heart pump. Danvers, Mass.-based Abiomed designed the Impella ECP heart pump to be compatible with small-bore access and closure techniques. It measures 3 mm in diameter when inserted and removed from the … [Read more...] about FDA grants breakthrough device designation for Abiomed’s Impella ECP heart pump
FDA approves Abbott Amplatzer Amulet to treat AFib
Abbott this week announced that it has received FDA approval for its Amplatzer Amulet device to treat atrial fibrillation. The Amplatzer Amulet is a left atrial appendage occluder for treating atrial fibrillation (AFib) with a risk of ischemic stroke. The device is a small pouch connected to the upper left chamber of the heart. Get the full … [Read more...] about FDA approves Abbott Amplatzer Amulet to treat AFib
Freudenberg Medical touts expanded catheter sensor integration capabilities
Freudenberg Medical recently announced that it has increased its catheter sensor integration capabilities. The expanded capabilities allow the company to work with sensors for temperature and pressure, entire sensor arrays and fiber optic sensors in catheter manufacturing. "At Freudenberg Medical, we understand the difficulty in handling … [Read more...] about Freudenberg Medical touts expanded catheter sensor integration capabilities
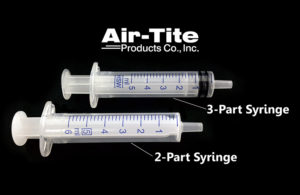
What is the difference between two-part and three-part syringes?
Syringes remain an integral part of the medical industry, yet they are often an afterthought in the R&D or production phase. William Foley, Air-Tite Products [Image courtesy of Air-Tite Products] With the wide variety of options available on the market containing fundamental differences in design and composition, can the syringe selection … [Read more...] about What is the difference between two-part and three-part syringes?
FDA clears Boston Scientific’s Exalt Model B single-use bronchoscope
Boston Scientific (NYSE:BSX) announced today that it received FDA 510(k) clearance for its Exalt Model B single-use bronchoscope. Marlborough, Mass.-based Boston Scientific designed the Exalt Model B for use in bedside procedures within the intensive care unit and operating room, according to a news release. Uses for the device include a wide … [Read more...] about FDA clears Boston Scientific’s Exalt Model B single-use bronchoscope