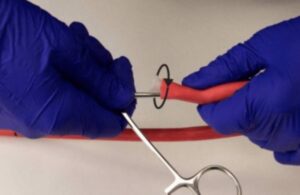
Atraverse Medical announced today that it closed a $29.4 million follow-on financing round to help accelerate growth. The San Diego-based company secured an oversubscribed seed funding round worth $12.5 million last year. This follow-on funding builds on that round, which helped to obtain FDA 510(k) clearance and begin the early rollout of … [Read more...] about Atraverse raises $29.4M for left-heart access device
CoreMap wins FDA IDE for ablation mapping tech
CoreMap announced that it received FDA investigational device exemption (IDE) to extend its electrophysiology (EP) mapping trial to the U.S. The study aims to evaluate the safety and effectiveness of the CoreMap endocardial EP mapping system in patients with AFib. It looks at both persistent AFib and long-standing persistent AFib. The company says … [Read more...] about CoreMap wins FDA IDE for ablation mapping tech
BD to begin real-world atherectomy registry for PAD
BD [WtwhTicker symbol="BDX"](NYSE: BDX)[/WtwhTicker] announced today that it plans to initiate a patient data registry for its Rotarex atherectomy system. The registry aims to measure real-world outcomes for patients with peripheral artery disease (PAD). XTRACT, a multi-center, single-arm, post-market registry assesses Rotarex's clinical … [Read more...] about BD to begin real-world atherectomy registry for PAD
BD warns on esophagogastric balloon tamponade tubes after death
The FDA issued a notice highlighting a letter issued by BD and its C.R. Bard Urology and Critical Care subsidiary warning of new balloon device instructions. This warning follows one death associated with the device issue. BD sent affected customers a notice updating instructions for all lots of certain esophagogastric balloon tamponade tubes. … [Read more...] about BD warns on esophagogastric balloon tamponade tubes after death
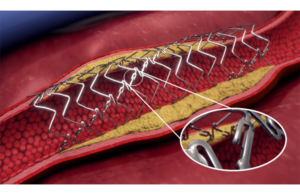
Elixir Medical reports sustained durability with bioadaptor compared to Medtronic stent
Elixir Medical announced three-year results from a large randomized controlled trial of its DynamX coronary bioadaptor system. The 445-patient BIOADAPTOR trial compares DynamX to the standard of care Medtronic Resolute Onyx drug-eluting stent (DES). It took place across 34 centers in Japan, Europe and New Zealand. Data — shared this week at … [Read more...] about Elixir Medical reports sustained durability with bioadaptor compared to Medtronic stent
Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Medtronic announced today that it received CE mark for several expanded indications for its Prevail balloon catheter. The expanded indications for the paclitaxel-coated percutaneous transluminal coronary angioplasty (PTCA) balloon catheter (or drug-coated balloon/DCB) cover the treatment of coronary artery disease (CAD). According to the medtech … [Read more...] about Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Johnson & Johnson MedTech launches ultrasound catheter for imaging in cardiac ablation procedures
Johnson & Johnson MedTech (NYSE: JNJ) announced today that it launched the SoundStar Crystal ultrasound catheter. The company launched the catheter in the U.S. for intracardiac echocardiography (ICE) imaging in cardiac ablation procedures. SoundStar Crystal provides clearer, enhanced image quality compared to other ICE catheters, according … [Read more...] about Johnson & Johnson MedTech launches ultrasound catheter for imaging in cardiac ablation procedures
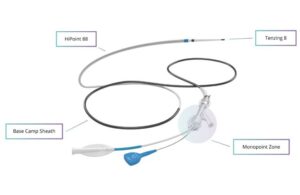
Route 92 Medical wins FDA nod for reperfusion system, reports positive study findings
Route 92 Medical announced today that it received FDA 510(k) clearance for its HiPoint reperfusion system. San Mateo, California-based Route 92’s HiPoint system includes the HiPoint 88 system powered by Tenzing 8, featuring the Monopoint approach. The company says it became the first neurovascular intervention company to win clearance for direct … [Read more...] about Route 92 Medical wins FDA nod for reperfusion system, reports positive study findings
J&J’s Shockwave touts study highlighting the use of IVL-first strategy for women
Johnson & Johnson MedTech‘s Shockwave Medical unit today announced study results highlighting the use of its IVL treatment in female patients. Shockwave announced 30-day primary endpoint results from the EMPOWER CAD study of its intravascular lithotripsy (IVL) therapy. It marks the first prospective, real-world percutaneous … [Read more...] about J&J’s Shockwave touts study highlighting the use of IVL-first strategy for women
Cordis launches 10,000-patient registry for drug-eluting balloon
Cordis today announced the initiation of a global registry to track real-world outcomes for the Selution SLR drug-eluting balloon (DEB). Miami Lakes, Florida-based Cordis expects the SELUTION Global Coronary Registry to enroll up to 10,000 patients worldwide. It says that would make it one of the largest coronary DEB registries to … [Read more...] about Cordis launches 10,000-patient registry for drug-eluting balloon