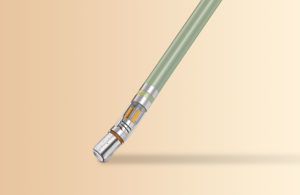
Partnering with a vertically integrated manufacturer can help engineers optimize designs, improve manufacturability and accelerate new product development. Michael Holt, Integer (Image courtesy of Integer) Medtech design engineers face extraordinary opportunities and challenges created by rapidly advancing clinical markets. To … [Read more...] about 6 ways the right tubing supplier can improve product development
Technologies & Devices
Heraeus Medical Components acquires Pulse Systems
Heraeus Medical Components announced this week that it has acquired Pulse Systems, a precision laser processor and manufacturer of nitinol and other metal-based implants and delivery systems. A key reason for the acquisition is the increasing importance of vascular access and delivery systems to enable new therapies, according to Peter Reimer, … [Read more...] about Heraeus Medical Components acquires Pulse Systems
Medtronic has a Class I recall involving balloon catheters to treat heart defects
FDA today designated a Medtronic recall of Rashkind balloon septostomy catheters as Class I, its most serious level. The balloon catheters create an atrial septal defect or to enlarge an existing atrial septal defect in order to treat congenital heart defects. Medtronic (NYSE:MDT) initiated a recall of the Rashkind balloon septostomy … [Read more...] about Medtronic has a Class I recall involving balloon catheters to treat heart defects
Syneo sold to investment firm
Private equity firm Arcline Investment Management announced that it has acquired a controlling stake in Syneo from Rockwood Equity. Syneo is a vertically-integrated machine, tooling and services company focused on the interventional medtech industry as well as specialty “press-fit” printed circuit board applications. Syneo has thousands of … [Read more...] about Syneo sold to investment firm
Philips touts post-stent imaging study results
Royal Philips (NYSE:PHG) announced today that one-year results of its Define PCI study that reported improved outcomes and less recurrent angina. The Define PCI study assessed the level of residual ischemia found in patients following a percutaneous coronary intervention (PCI) using a blinded instant wave-free ratio (iFR) pullback measurement, … [Read more...] about Philips touts post-stent imaging study results
J&J wins FDA approval for ablation catheter
Johnson & Johnson (NYSE:JNJ) recently announced that its Biosense Webster business has secured FDA approval for its Thermocool SmartTouch SF ablation catheter. The Thermocool SmartTouch received approval for the treatment of persistent atrial fibrillation, J&J announced yesterday. The approval came after results from the 381-patient, … [Read more...] about J&J wins FDA approval for ablation catheter
NanoVibronix device gains FDA nod for import to U.S.
NanoVibronix (NSDQ:NAOV) announced that it received FDA enforcement discretion for the U.S. distribution of the UroShield device. According to a news release, the FDA exercised its enforcement discretion, giving Elmsford, N.Y.-based NanoVibronix’s UroShield an intended use code (IUC), clearing the way for the import of the system to the U.S. … [Read more...] about NanoVibronix device gains FDA nod for import to U.S.
Boston Scientific warns on Hurricane RX biliary balloon dilatation catheter
Boston Scientific (NYSE:BSX) said it is recalling specific lots/batches of its Hurricane RX biliary balloon dilation catheters. Marlborough, Mass.-based Boston Scientific, which issued a warning letter from its international location in France, said the removal comes in response to an increase in complaints of the RX tunnel component (black … [Read more...] about Boston Scientific warns on Hurricane RX biliary balloon dilatation catheter
BioProtect closes $25M Series D equity financing round
BioProtect this week announced it closed a $25 million Series D equity financing round. Peregrine Ventures and an unnamed strategic investor completed the financing round that was led by Almeida Ventures and Vincent Tchenguiz, chairperson of Consensus Business Group. KB Investments of South Korea and Triventures also participated in the … [Read more...] about BioProtect closes $25M Series D equity financing round
Acutus Medical 3D imaging and mapping catheter wins FDA clearance
Acutus medical recently won FDA 510(k) clearance of its AcQMap 3D imaging and mapping catheter. The mapping catheter builds on its predecessor and improves handling and deliverability. It combines 48 ultrasound transducers responsible for creating anatomical geometry and 48 engineered electrodes that enable electrical activation patterns to … [Read more...] about Acutus Medical 3D imaging and mapping catheter wins FDA clearance