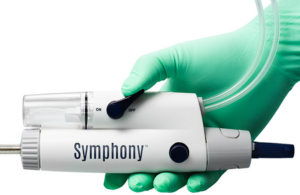
Imperative Care today announced FDA 510(k) clearance of its Symphony 16 Fr 82cm catheter for venous thrombosis and announced the first successful cases. Campbell, California-based Imperative Care said the catheter joins the Symphony Thrombectomy System's 16 Fr 117 cm and 24 Fr 85 cm catheters. "The customized length of the 16 Fr 82 cm … [Read more...] about FDA clears Imperative Care’s Symphony 16F 82cm catheter
Jabil partners with Midwest Interventional Systems on catheter tech
Jabil and Midwest Interventional Systems (MIS) have entered into a collaborative agreement that will expand what the two contract development and manufacturing organizations can do for medical device developers, including those working on minimally invasive systems. St. Petersburg, Florida-based Jabil and Maple Grove, Minnesota-based MIS said … [Read more...] about Jabil partners with Midwest Interventional Systems on catheter tech
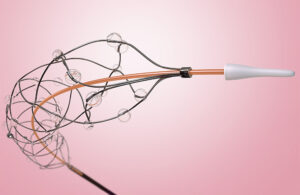
First Look: Flow Medical’s next-generation thrombolysis catheter for pulmonary embolism
Flow Medical is developing a thrombolysis catheter to improve the treatment of blood clots in pulmonary embolism patients. The Chicago-based startup designed its minimally invasive system with a novel nitinol scaffold that perforates blood clots and infuses them with tissue plasminogen activator (TPA), a thrombolytic drug commonly referred to as … [Read more...] about First Look: Flow Medical’s next-generation thrombolysis catheter for pulmonary embolism
Boston Scientific resumes study of pulse field ablation as first-line AFib therapy
Boston Scientific (NYSE: BSX) is resuming its “Avant Guard” study of pulse field ablation as a first-line therapy for persistent atrial fibrillation (AFib). The device developer paused the trial last month, with CEO Mike Mahoney citing the need to “assess a few unanticipated observations.” Boston Scientific said it did not halt the trial due to … [Read more...] about Boston Scientific resumes study of pulse field ablation as first-line AFib therapy
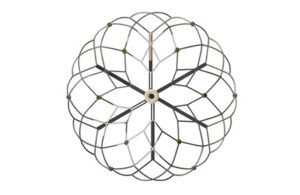
Pulsed field ablation catheters take shape at Medtronic, Boston Scientific, Abbott and J&J’s Biosense Webster
Experts from Medtech Big 100 device developers — Medtronic, Johnson & Johnson MedTech, Abbott and Boston Scientific — discuss the various shapes of their pulsed field ablation (PFA) catheters. From flowers and loops to globes, baskets and balloons, there's no standard shape for the pulsed field ablation (PFA) catheters coming out of Medtronic, … [Read more...] about Pulsed field ablation catheters take shape at Medtronic, Boston Scientific, Abbott and J&J’s Biosense Webster
How Medtronic designed the PulseSelect pulsed field ablation system for AFib
Medtronic‘s PulseSelect became the first pulsed field ablation (PFA) system to win FDA approval for treating atrial fibrillation (AFib) in December 2023. The world’s largest medical device manufacturer achieved that milestone about 15 years after one of its engineers, Mark Stewart, coined the term “pulsed field ablation” in a research paper … [Read more...] about How Medtronic designed the PulseSelect pulsed field ablation system for AFib
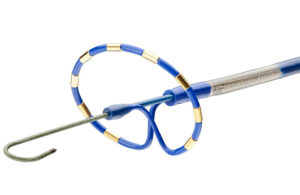
This medtech developer is throwing everything at clots
Retriever Medical is developing a thrombectomy system with a four-front approach for treating blood clots. The Las Vegas-based startup calls its technology VORS AMD, for vascular occlusion removal system with aspiration, mechanical and drug delivery. The approach combines nitinol baskets for mechanical thrombectomy with aspiration, clot-busting … [Read more...] about This medtech developer is throwing everything at clots
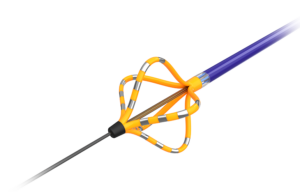
First look: Medtronic’s Sphere-360 pulsed field ablation catheter design has some new tricks
The investigational Medtronic Sphere-360 pulsed field ablation and mapping catheter uses nitinol for a feature that's unique within Medtronic's PFA device portfolio.
Medtronic recently offered a first look at its Sphere-360 pulsed field ablation (PFA) and mapping catheter, an investigational device with some design features that are new for the world's largest device manufacturer.
In an interview with Medical Design & Outsourcing, Tim Laske, VP of research and business development for Medtronic Cardiac … [Read more...] about First look: Medtronic’s Sphere-360 pulsed field ablation catheter design has some new tricks
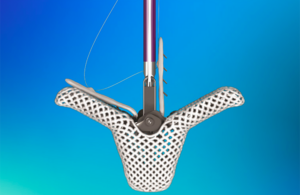
Nitinol grips prevent slips in Abbott’s heart valve clips
Nitinol is a key material in the heart valve clips that Abbott designed for its TriClip and MitraClip transcatheter edge-to-edge repair (TEER) systems. Abbott designed the TriClip system (approved by the FDA in April 2024) for reducing tricuspid valve regurgitation using fourth-generation heart valve clips that Abbott originally developed for … [Read more...] about Nitinol grips prevent slips in Abbott’s heart valve clips
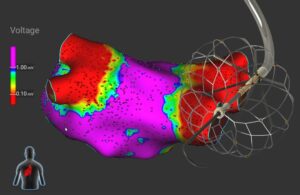
High voltage in the heart: PFA catheter design tips from Biosense Webster
Tushar Sharma at J&J Medtech’s Biosense Webster discusses pulsed-field ablation (PFA) catheter shapes, sensors, electrodes and device design tips.
Biosense Webster’s pulsed-field ablation (PFA) catheter design and development starts in the heart.
That’s where PFA treats atrial fibrillation (AFib) by killing cardiac cells to isolate errant heart signals and prevent them from triggering irregular heartbeats. PFA uses electric fields to permanently open holes in the walls of heart cells … [Read more...] about High voltage in the heart: PFA catheter design tips from Biosense Webster