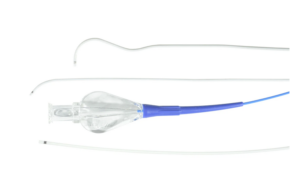
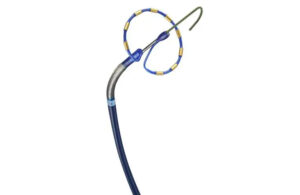
Merit Medical Systems this week announced the launch of its expanded Maestro microcatheter product line. The Maestro product line features embolotherapy devices, which include Embosphere Microspheres. The new 165 cm Maestro length will be available in 2.1F, 2.4F, 2.8F and 2.9F catheter diameters to support a broad range of embolization … [Read more...] about Merit Medical launches expanded Maestro microcatheter line
LeMaitre Vascular shares rise on 130% profit gains in Q2
LeMaitre Vascular (Nasdaq:LMAT) this week posted second-quarter results that beat the overall consensus on Wall Street. The Burlington, Massachusetts-based company reported profits of $8.1 million, or 36¢ per share, on sales of $50.1 million, for a sales growth of 19.02% and profit gains of 130.4% compared to Q3 2022. Earnings per share were … [Read more...] about LeMaitre Vascular shares rise on 130% profit gains in Q2
Surmodics more than doubles sales in Q3
Surmodics (Nasdaq:SRDX) this week announced third-quarter results that beat the overall consensus on Wall Street. The Eden Prairie, Minnesota-based company reported profits of $7.3 million, or 52¢ per share, on sales of $52.5 million for the three months ended June 30. Compared to Q3 2022, the company had a sales gain of 111.2% and profit gains … [Read more...] about Surmodics more than doubles sales in Q3
Access Vascular’s HydroPICC shows positive results in reducing clot formation and failures
Access Vascular this week announced positive results from a study of its HydroPICC catheter. Billerica, Massachusetts-based Access Vascular designed the device to help reduce the most common and costly complications that are associated with vascular access procedures, including infection, thrombosis and phlebitis. The HydroPICC is constructed of … [Read more...] about Access Vascular’s HydroPICC shows positive results in reducing clot formation and failures
Freudenberg Medical implements VR in training, reduces scrap materials
Freudenberg Medical recently announced it implemented virtual reality training for employees working in catheter production at operations in Ireland and the U.S. The VR training program will help ensure employees have the necessary skills to meet the high-quality standards in catheter manufacturing for customers worldwide, according to the … [Read more...] about Freudenberg Medical implements VR in training, reduces scrap materials
How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
Tim Laske, VP of research and business development for Medtronic‘s cardiac ablation solutions business, discusses the challenges of designing devices for the heart and explores the properties of nitinol. The heart is often one of the most underappreciated aspects of human anatomy, and its atrial appendages are often overlooked even in cardiac … [Read more...] about How Medtronic uses nitinol to improve the structure and effectiveness of heart devices
Gore enrolls first patient in Gore ViaFort vascular stent iliofemoral study
Gore this week announced it enrolled the first U.S. patient in its pivotal study for its ViaFort vascular stent. The prospective, non-randomized, multi-center, single-arm study will have a five-year follow-up and will evaluate the investigational Gore ViaFort vascular stent for the treatment of symptomatic iliofemoral venous obstruction. It will … [Read more...] about Gore enrolls first patient in Gore ViaFort vascular stent iliofemoral study
VitalPath appoints medtech industry veteran Andrew Holman as CEO
VitalPath today announced it appointed Andrew Holman as its new CEO. Holman brings more than 28 years of medical device experience to the complex catheter solution company. He has held numerous leadership roles at contract manufacturers and OEMs. Most recently, he was the chief commercial officer at Prytime Medical Devices, where he helped bring … [Read more...] about VitalPath appoints medtech industry veteran Andrew Holman as CEO
Teleflex launches two new PICC insertion devices
Teleflex (NYSE:TFX) today announced it launched two new devices to enhance PICC insertion procedures and reduce complications. The company launched the Arrow VPS Rhythm DLX device and the NaviCurve stylet, both of which Teleflex designed to work together to give vascular access specialists more efficient and predictable PICC placement. VPS … [Read more...] about Teleflex launches two new PICC insertion devices
Surmodics enrolls first patient in Pounce thrombectomy system registry study
Surmodics recently announced it enrolled the first patient in its Prowl registry study of its Pounce thrombectomy system. Prowl is an open-label, retrospective, multi-center, U.S. registry of the Surmodics Pounce system for the non-surgical removal of emboli and thrombi in the peripheral arterial vasculature. The registry aims to collect … [Read more...] about Surmodics enrolls first patient in Pounce thrombectomy system registry study