FujiFilm today announced it received FDA 510(k) clearance for its Scale Eye endoscopic imaging technology. Scale Eye is part of the company's expanding Eluxeo endoscopic imaging system. It has a laser-equipped colonoscope and endoscopy support software that displays linear or circular vital measurements or scales on an endoscopy monitor. The … [Read more...] about FDA clears FujiFilm’s Scale Eye endoscopic imaging tech
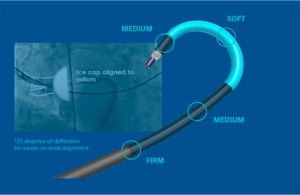
Boston Scientific warns on fragment embolization in some PolarSheath devices
Boston Scientific has issued an urgent field safety notice for some of its PolarSheath steerable sheaths due to the risk of embolization. The company is removing specific batches of PolarSheath steerable sheaths due to a tooling error in manufacturing, which may have caused delimitation of the inner lumen of the sheath shaft in a subset of … [Read more...] about Boston Scientific warns on fragment embolization in some PolarSheath devices
Vena Medical achieves first-in-patient for its MicroAngioscope
Vena Medical this week announced that physicians at The Ottawa Hospital (TOH) were the first to use its stroke technology, the MicroAngioscope. Dr. Robert Fahed, an interventional neuroradiologist at TOH, used the MicroAngioscope to treat a patient who had repeated strokes. According to TOH, the device's advanced and high-resolution imaging … [Read more...] about Vena Medical achieves first-in-patient for its MicroAngioscope
FDA clears AngioDynamics’ Auryon XL catheter
AngioDynamics announced today that it received FDA 510(k) clearance for its Auryon XL catheter for use with the Auryon atherectomy system in treating peripheral arterial disease (PAD). The Auryon XP catheter is a 225 cm radial access catheter available in 0.9 mm and 1.5 mm diameters to expand treatment access points in atherectomy procedures in … [Read more...] about FDA clears AngioDynamics’ Auryon XL catheter
The worst catheter-based device recalls of 2023
The U.S. saw seven severe catheter-based device recalls in 2023, according to the FDA's medical device recall database. The agency in 2023 tagged 61 medical device recalls as Class I — the most serious level. For comparison, the FDA recalled 60 devices in 2022, of which four were related to catheter-based devices. Last year, the most serious … [Read more...] about The worst catheter-based device recalls of 2023
First patients treated using Stereotaxis MAGiC ablation catheter
Stereotaxis today announced the first patients have been successfully treated using its MAGiC magnetic interventional ablation catheter. The MAGiC catheter is a robotically navigated magnetic ablation catheter that performs minimally invasive cardiac ablation procedures. The first procedures were performed in Lithuania in eight patients … [Read more...] about First patients treated using Stereotaxis MAGiC ablation catheter
Catheter-based innovations we can’t stop thinking about
Catheter-based devices had their year in 2023. Medtech companies set their sights on improving patient care in cardiology, neurology and more. Our previous roundup of the top 10 most-read catheter-based news stories of 2023 wasn't enough for us. Medical Tubing + Extrusion's editors could not stop thinking about several other stories when … [Read more...] about Catheter-based innovations we can’t stop thinking about
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
Recor Medical launches renal denervation system, announces first commercial cases
Recor Medical announced the first commercial uses of its Paradise ultrasound renal denervation (uRDN) system for treating hypertension. The first commercial uses follow the company’s landmark FDA approval for Paradise last week. Approval allows for the use of Paradise as an adjunctive treatment option when lifestyle changes and … [Read more...] about Recor Medical launches renal denervation system, announces first commercial cases
Olympus launches Evis X1 endoscopy system
Olympus recently announced it launched the Evis X1 endoscopy system in the U.S. The Evis X1 received FDA clearance earlier this year with two compatible endoscopes, the GIF-1100 gastrointestinal videoscope and the CF-HQ1100DL/I colonovideoscope. It is indicated for use in the upper digestive tract and the lower digestive tract. Evis X1 has an … [Read more...] about Olympus launches Evis X1 endoscopy system