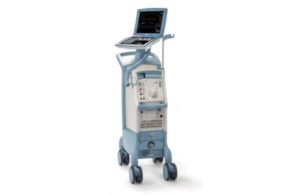
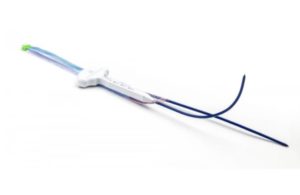
The FDA today issued a notice confirming that the recall of Medtronic’s (NYSE:MDT) HawkOne system is Class I, the most serious kind. So far in 2022, the FDA has classified three Medtronic-related recalls as Class I, with the Covidien (part of Medtronic) Puritan Bennett 980 series ventilator and the Synergy Cranial and StealthStation S7 Cranial … [Read more...] about Medtronic’s recall of HawkOne system is Class I
Alcon closes Ivantis acquisition
Alcon (NYSE:ALC) announced today that it completed its $475 million acquisition of glaucoma surgery device maker Ivantis. Geneva, Switzerland-based Alcon initially announced the acquisition of Ivantis, which develops the Hydrus Microstent minimally invasive glaucoma surgery (MIGS) device, in November 2021. According to a news release, the … [Read more...] about Alcon closes Ivantis acquisition
Medtronic to acquire cardiac arrhythmia treatment developer Affera
Medtronic (NYSE:MDT) announced today that it agreed to acquire cardiac mapping and ablation technology developer Affera. Newton, Massachusetts-based Affera designs and manufactures cardiac mapping and navigation systems, as well as catheter-based cardiac ablation technologies, including a differentiated, focal pulsed-field ablation platform for … [Read more...] about Medtronic to acquire cardiac arrhythmia treatment developer Affera
VitalPath acquires Modern Catheter Technologies
VitalPath announced today that it acquired catheter delivery system developer Modern Catheter Technologies. The Roseville, Minnesota-based company said in a news release that the acquisition builds upon its acquisition of VitalDyne in March 2021. Get the full story at our sister site, Medical Design & Outsourcing. … [Read more...] about VitalPath acquires Modern Catheter Technologies
Laborie acquires exclusive license for drug-coated baloon treatment for urethral strictures
Laborie Medical Technologies announced today that it acquired an exclusive license to the Optilume urethral drug-coated balloon (DCB). Portsmouth, New Hampshire-based Laborie purchased a perpetual, exclusive license to Optilume — developed by Urotronic — following FDA approval in December 2021. The DCB platform received CE mark approval for use … [Read more...] about Laborie acquires exclusive license for drug-coated baloon treatment for urethral strictures
Getinge intra-aortic balloon pump recall is Class I
The FDA determined that Getinge's recall of its Cardiosave Hybrid and CardioSave Rescue devices is Class I, the most serious kind. Datascope/Getinge/Maquet designed the CardioSave Hybrid and CardioSave Rescue intra-aortic balloon pumps (IABP) as cardiac assist devices for patients undergoing cardiac and non-cardiac surgery. They are used in … [Read more...] about Getinge intra-aortic balloon pump recall is Class I
CereVasc successfully performs first-in-human procedure with eShunt system
CereVasc announced today that it completed the first treatment with its eShunt system for treating communicating hydrocephalus (CH). Boston-based CereVasc touts the eShunt as the first minimally invasive cerebrospinal fluid shunt and delivery technology designed to avoid the need for invasive surgery and extended hospitalization associated with … [Read more...] about CereVasc successfully performs first-in-human procedure with eShunt system
CardioMech reports first in-person procedure with its transcatheter mitral valve repair tech
CardioMech confirmed the completion of the first procedure with its transcatheter mitral valve repair (TMVR) technology. At the Sanger Heart & Vascular Institute in North Carolina, a multidisciplinary heart team evaluated a patient diagnosed with severe symptomatic degenerative mitral regurgitation (DMR). They offered the patient a choice: … [Read more...] about CardioMech reports first in-person procedure with its transcatheter mitral valve repair tech
Caldera launches new Desara TVez for stress urinary incontinence
Surgical product developer Caldera Medical announced today that it launched the Desara TVez as part of its Desara portfolio. Los Angeles-based Caldera’s Desara family of products includes offerings for treating stress urinary incontinence, pelvic organ prolapse and polyps. Get the full story on our sister site, MassDevice. … [Read more...] about Caldera launches new Desara TVez for stress urinary incontinence
BD acquires Venclose and its RF ablation technology
BD (NYSE:BDX) announced today that it acquired Venclose and its solutions for treating chronic venous insufficiency (CVI). CVI occurs as the result of malfunctioning valves and can lead to varicose veins. Venclose’s CVI treatment uses radio frequency (RF) ablation technology, with its catheters potentially reducing post-operative pain and … [Read more...] about BD acquires Venclose and its RF ablation technology