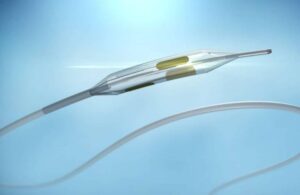
Metavention announced today that the FDA granted approval to initiate an investigational device exemption (IDE) trial for its renal denervation system. Minneapolis-based Metavention designed its integrated radiofrequency (iRF) renal denervation system to treat hypertension. The company plans for its randomized, double-blinded, sham-controlled … [Read more...] about FDA approves Metavention’s renal denervation for hypertension pivotal IDE study
FDA approves Surmodics SurVeil drug-coated balloon
Surmodics (Nasdaq:SRDX) announced today that the FDA granted approval for its SurVeil drug-coated balloon (DCB). Eden Prairie, Minnesota–based Surmodics may now market and sell SurVeil in the U.S. for percutaneous transluminal angioplasty. Use follows appropriate vessel preparation or de novo or restenotic lesions in femoral and popliteal … [Read more...] about FDA approves Surmodics SurVeil drug-coated balloon
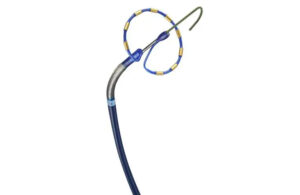
FDA clears Pounce LP thrombectomy system from Surmodics
Surmodics (Nasdaq:SRDX) today announced that it received FDA 510(k) clearance for its Pounce low-profile (LP) thrombectomy system. News of the regulatory nod comes just one day after Needham analysts upgraded Surmodics stock from "Hold" to "Buy." The analysts cited positive developments pointing toward the likely approval of Surmodics' SurVeil … [Read more...] about FDA clears Pounce LP thrombectomy system from Surmodics
Confluent Medical now offers EU MDR-compliant polyimide tubing
Confluent Medical Technologies announced today that it now offers REACH and EU MDR-compliant polyimide tubing. REACH, a European Union regulation, imposes stringent limitations for the EU market. It covers the amount of restricted substances contained in devices sold in the EU. It stands for Registration, Evaluation, Authorization, and … [Read more...] about Confluent Medical now offers EU MDR-compliant polyimide tubing
Merit Medical spends $132.5M on acquisitions to expand its offerings
AngioDynamics (Nasdaq:ANGO) announced that it completed the sale of its dialysis product portfolio to Merit Medical Systems. It was part of a series of product acquisitions worth $132.5 million that Merit (Nasdaq: MMSI) has announced. Latham, New York-based AngioDynamics sold its dialysis portfolio and the BioSentry product for $100 million … [Read more...] about Merit Medical spends $132.5M on acquisitions to expand its offerings
Olympus to establish series of digital centers based on Odin Vision’s cloud-AI tech
Olympus announced that it plans to establish a series of digital excellence centers (DECs) following its acquisition of Odin Vision. London-based Odin Vision develops a portfolio of commercially available computer-aided detection/diagnostic (CAD) solutions. It also holds an innovation pipeline of cloud-enabled applications. Olympus agreed to … [Read more...] about Olympus to establish series of digital centers based on Odin Vision’s cloud-AI tech
BD expands availability of all-in-one flush pre-filled syringe
BD (NYSE:BDX) announced today that it expanded customer availability for its all-in-one pre-filled flush syringe. Franklin Lakes, New Jersey-based BD designed its PosiFlush SafeScrub syringe with an integrated disinfection unit. It reinforces compliance to infection prevention guidelines, simplifying nursing workflow. BD built the active … [Read more...] about BD expands availability of all-in-one flush pre-filled syringe
Medtronic reports positive study results for PulseSelect pulsed-field ablation system
Medtronic (NYSE:MDT) today announced positive findings from a secondary analysis of the PULSED AF study for its pulsed-field ablation technology. The analysis demonstrated positive results for the PulseSelect system, including atrial arrhythmia (AA) burden reduction. This correlated to both improved quality of life and decreased healthcare … [Read more...] about Medtronic reports positive study results for PulseSelect pulsed-field ablation system
Abbott reports positive data for TriClip TEER system
Abbott this week announced late-breaking data supporting the benefits of its TriClip transcatheter edge-to-edge repair (TEER) system. The company presented study data at EuroPCR in Paris. This study evaluated TriClip, a first-of-its-kind, minimally invasive device, in treating patients with leaky tricuspid valves. Abbott’s bRIGHT study … [Read more...] about Abbott reports positive data for TriClip TEER system
Data supports Biosense Webster RF ablation for treating AFib
Johnson & Johnson MedTech's Biosense Webster today announced data from a study of its QDOT Micro catheter for treating AFib. JACC: Clinical Electrophysiology published data from the Q-FFICIENCY study evaluating QDOT Micro's safety and 12-month effectiveness. The study observed use of the catheter in paroxysmal AFib ablation using a … [Read more...] about Data supports Biosense Webster RF ablation for treating AFib