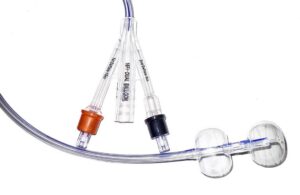
HR Pharmaceuticals announced that it entered into an exclusive commercial agreement with Poiesis Medical to license catheter technology. The deal centers around Poiesis' dual-balloon catheter, the Duette system. Under the terms, HR Pharmaceuticals has exclusive commercialization rights to Duette in North America. The deal expands HR … [Read more...] about HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical
J&J’s Acclarent wins FDA nod for pediatric ear tube balloon dilation system
Johnson & Johnson MedTech unit Acclarent announced that it won a new FDA clearance for its Aera Eustachian tube balloon dilation system. While Acclarent still belongs to Johnson & Johnson, Integra Lifesciences is set to buy it for $275 million next year. While the completion of that deal awaits, the company cleared a regulatory hurdle … [Read more...] about J&J’s Acclarent wins FDA nod for pediatric ear tube balloon dilation system
FDA clears neurovascular aspiration, access catheters from Perfuze
Perfuze announced today that the FDA cleared its Millipede 070 aspiration catheter and second-generation Millipede 088 access catheter. Galway, Ireland-based Perfuze developed Millipede 070 to address critical unmet needs in ischemic stroke treatment. It aims to remove clots rapidly and safely through a novel, unique catheter. Millipede 070 … [Read more...] about FDA clears neurovascular aspiration, access catheters from Perfuze
Pulse Biosciences announces first human use of pulsed-field ablation catheter
Pulse Biosciences (Nasdaq:PLSE) today announced the completion of the first-in-human procedures for its CellFX nsPFA cardiac catheter. The Hayward, California-based company completed the first five procedures in its first-in-human feasibility study. Treating physicians successfully discharged all patients. They'll continue to be monitored and … [Read more...] about Pulse Biosciences announces first human use of pulsed-field ablation catheter
Olympus bronchoscopes in Class I recall could lead to burns, fire
The FDA labeled a recall of Olympus bronchoscopes that could lead to burns or fire Class I, the most serious kind. Olympus reports 192 complaints related to the issue, including four injuries. The company has received no reports of death. This recall — a correction, not a product removal — applies to Olympus bronchofiberscopes and … [Read more...] about Olympus bronchoscopes in Class I recall could lead to burns, fire
B. Braun launches luer device for IV infusions
B. Braun Medical announced that it launched its new CareSite Micro luer access device designed to make IV access safer. Bethlehem, Pennsylvania-based B. Braun designed the device to reduce exposure to harmful chemicals. It aims to decrease infection risk in IV access for patients and healthcare providers. The company says its device is the … [Read more...] about B. Braun launches luer device for IV infusions
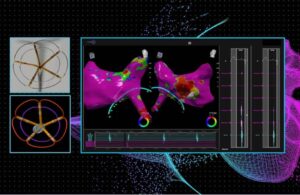
J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Johnson & Johnson's Biosense Webster today announced the first completed patient cases in a study of its dual-energy ablation catheter. Biosense Webster designed the ThermoCool SmartTouch SF to deliver both radiofrequency (RF) and pulsed-field ablation (PFA) energy. The SmartPulse pivotal study evaluates the dual-energy system in the … [Read more...] about J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Micro Medical Solutions completes enrollment in vascular stent study
Micro Medical Solutions announced today that it completed enrollment in a U.S. pivotal clinical study of its MicroStent system. Wilmington, Massachusetts-based Micro Medical set up its study to evaluate MicroStent’s safety and effectiveness. The study pits the stent against the current standard of care, percutaneous transluminal angioplasty … [Read more...] about Micro Medical Solutions completes enrollment in vascular stent study
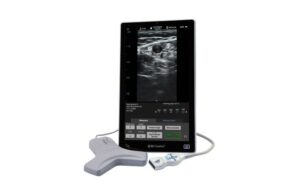
BD launches SiteRite vascular access ultrasound system
BD (NYSE:BD) announced today that it launched its new SiteRite 9 advanced ultrasound system for helping with vascular access device placement. Franklin Lakes, New Jersey-based BD designed the system to help improve clinician efficiency when placing these devices. They include peripherally inserted central catheters (PICCs), central venous … [Read more...] about BD launches SiteRite vascular access ultrasound system
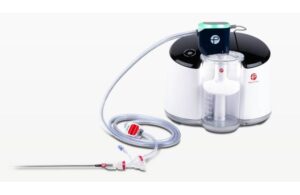
First patient enrolled in Penumbra study of computer-assisted vacuum thrombectomy
Penumbra (NYSE:PEN) today announced the first patient enrolled in STORM-PE, a trial of its Lightning Flash thrombectomy system. Lightning Flash, a mechanical thrombectomy system, received FDA clearance in January. The system features Penumbra’s novel Lightning intelligent aspiration technology with dual-clot detection algorithms. Combining it … [Read more...] about First patient enrolled in Penumbra study of computer-assisted vacuum thrombectomy