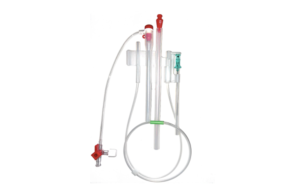
Stratus Medical this week said it received CE mark approval for its Nimbus RF multitined expandable electrode and Vesta RF cannula. The Nimbus multitined expandable electrode is designed to create a large 8 mm to 10 mm prolate spheroid lesion for pain relief. More than 100,000 of the devices have been used to treat chronic pain in patients in … [Read more...] about Stratus Medical receives CE mark for Nimbus RF multitined expandable electrode, Vesta RF cannula
Product Development
Micro Medical Solutions wins FDA breakthrough device designation for vascular stent
Micro Medical Solutions today said it has received FDA breakthrough device designation for its MicroStent vascular stent. The stent is designed to maintain vessel patency, enhance wound treatment and improve quality of life and blood flow to reduce amputations and mortality for patients with critical limb-threatening ischemia (CLTI) from … [Read more...] about Micro Medical Solutions wins FDA breakthrough device designation for vascular stent
This device stops aorta blood flow to save lives
Stopping internal bleeding from torso trauma has its technological challenges. Asha Parekh, co-founder and CEO of Front Line Medical Technologies, has developed a small, quick and easy device that could solve the problem. Front Line Medical Technologies (London, Ontario) is an early-stage medical device company that is developing a way to stop … [Read more...] about This device stops aorta blood flow to save lives
Erbe Elektromedizin to acquire Maxer Endoscopy
Erbe Elektromedizin announced today that it is set to acquire Maxer Endoscopy for an undisclosed amount. Teubingen, Germany–based Erbe Elektromedizin develops, manufactures and distributes surgical instruments and devices in almost all specialist areas. Combined with other Erbe technologies, the surgical instruments are based on … [Read more...] about Erbe Elektromedizin to acquire Maxer Endoscopy
IceCure Medical wins FDA breakthrough device designation for cryoablation system
IceCure Medical today said it received FDA breakthrough device designation for its ProSense cryoablation system. ProSense is a liquid nitrogen-based cryoablation system that is a minimally invasive treatment for cancer tumors. It is designed for use in the treatment of TI invasive breast cancer or in patients not suitable for surgical … [Read more...] about IceCure Medical wins FDA breakthrough device designation for cryoablation system
4 innovative devices delivered through catheters
Physicians use catheters to deploy numerous life-saving devices across the healthcare realm, from cardiovascular to neurovascular. They typically insert the thin, flexible tubes in a process called catheterization to deploy devices such as left ventricular assist devices (LVADs), transcatheter aortic valve replacements (TAVRs) and occluders to … [Read more...] about 4 innovative devices delivered through catheters
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
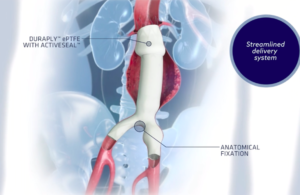
Cook Medical wins FDA breakthrough device designation for next-gen endovascular graft
Cook Medical announced that FDA has granted breakthrough device designation for its Zenith Fenestrated+ endovascular graft (ZFEN+), the next-gen version of its Zenith Fenestrated AAA endovascular graft. The designation — a first for Bloomington, Ind.–based Cook Medical — will enable priority review and better communication with FDA during the … [Read more...] about Cook Medical wins FDA breakthrough device designation for next-gen endovascular graft
NewAge Industries announces expansion, plans another
Plastic and silicone tubing manufacturer NewAge Industries (Southampton, Pa.) expanded into a recently vacated warehouse at its headquarters in Bucks County, Pennsylvania. The company said it has experienced significant growth over the past several years and needed more room for its inventory of tubing, reinforced hose, custom molded products, and … [Read more...] about NewAge Industries announces expansion, plans another
Cardiovascular Systems, Chansu Vascular Technologies partner to develop drug-coated balloons
Cardiovascular Systems recently announced that it has partnered with Chansu Vascular Technologies to develop a new drug-coated balloon technology. Through the partnership, Cardiovascular Systems and Chansu Vascular Technologies will develop peripheral and coronary everolimus drug-coated balloons. Cardiovascular Systems will provide … [Read more...] about Cardiovascular Systems, Chansu Vascular Technologies partner to develop drug-coated balloons