This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
Product Development
The 24 best medical device innovations of 2022
The Galien Foundation recently announced its nominees of medical device innovations for its 2022 Prix Galien USA awards. There are 24 medical technologies nominated for the annual award this year, up from 18 nominees in 2021. The Galien Foundation’s annual Prix Galien awards highlight devices, biotechnology and pharmaceutical products … [Read more...] about The 24 best medical device innovations of 2022
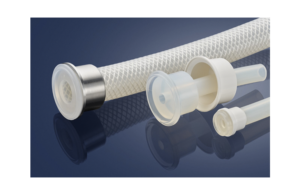
Freudenberg Medical launches HelixTC bioprocessing overmolded silicone ends
Freudenberg today launched its HelixTC silicone overmolded sanitary ends for bioprocessing fluid transfer applications. HelixTC is based on Freudenberg's PharmaFocus Premium product line of platinum-cured silicone tubing. It is an alternative to standard barbed TC connections. The new overmolded sanitary ends are available in standard or mini … [Read more...] about Freudenberg Medical launches HelixTC bioprocessing overmolded silicone ends
Design challenges to overcome when developing cardiac ablation devices
It’s about figuring out how and where to go with the cardiac ablation and then engineering the best catheter-based delivery device, according to a top Acutus Medical scientist. Cardiac ablation is when a physician intentionally destroys a small amount of tissue in the heart to treat and prevent heart rhythm problems. The procedure creates … [Read more...] about Design challenges to overcome when developing cardiac ablation devices
What is pulsed-field ablation? Here’s what you need to know
Top experts at Boston Scientific, Medtronic and Acutus Medical shared insights about pulsed-field ablation’s potential at DeviceTalks Boston. Pulsed-field ablation is a non-thermal method for cardiac ablation that has the potential to positively disrupt the way atrial fibrillation is treated. PFA’s roots go back to the dc ablation tech of the … [Read more...] about What is pulsed-field ablation? Here’s what you need to know
‘Itty bitty’ falloposcope imaging device used inside fallopian tubes for the first time
After years of development, University of Arizona researchers have captured their first images inside fallopian tubes with a new device that could be used to search for early signs of ovarian cancer. University of Arizona BIO5 Institute Director Jennifer Barton developed the high-resolution falloposcope, which has a diameter of only 0.8 … [Read more...] about ‘Itty bitty’ falloposcope imaging device used inside fallopian tubes for the first time
Plastics and medical devices: Changes for safety and cost
The move toward polypropylene and polyethylene in medical devices requires new manufacturing and assembly solutions such as ultrasonic and laser welding. Didier Perret, Emerson Plastics are ubiquitous in medical applications thanks to their light weight, durability and flexibility, among other attributes. However, concern has been increasing … [Read more...] about Plastics and medical devices: Changes for safety and cost
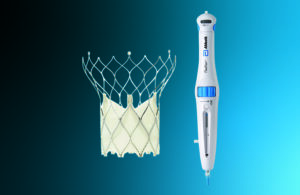
Abbott sees the delivery system as a differentiator for its TAVR
A top Abbott executive explains how the company's FlexNav delivery system could make the Portico TAVR competitive. Abbott is aiming to claw away market share from transcatheter aortic valve replacement pioneers Edwards Lifesciences and Medtronic. The Abbott Park, Illinois–based medtech company in September announced FDA approval of its Portico … [Read more...] about Abbott sees the delivery system as a differentiator for its TAVR
Raumedic plans U.S. headquarters expansion
Raumedic today said it intends to expand its clean room manufacturing facilities at its U.S. headquarters in Mills River, North Carolina. The new clean room is expected to be completed by February 2022 and used for new product lines. The Helmbrechts, Germany-based medical device component developer and manufacturer built its existing … [Read more...] about Raumedic plans U.S. headquarters expansion
Surmodics acquires Vetex Medical and its mechanical thrombectomy catheter
Surmodics (NSDQ:SRDX) announced that it acquired Vetex Medical, a developer and manufacturer of venous clot removal solutions. Buying Galway, Ireland–based Vetex Medical provides Surmodics a second FDA-cleared device for its thrombectomy portfolio. Vetex designed the ReVene thrombectomy catheter to remove large, mixed-morphology blood clots … [Read more...] about Surmodics acquires Vetex Medical and its mechanical thrombectomy catheter