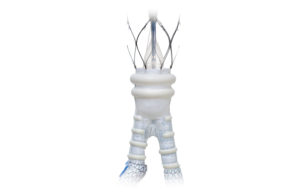
Boston Scientific this week announced positive data for its Eluvia drug-eluting vascular stent system. Data presented this week included one-year results from the company's Eminent trial, which showed superiority of the Eluvia stent compared to self-expanding bare metal stents (BMS) to treat patients with peripheral artery disease (PAD) and … [Read more...] about Boston Scientific announces positive data for Eluvia stent
Stents
Medtronic’s Pipeline Flex problems expand
The FDA today issued a notice confirming a Class I recall of the Pipeline Flex embolization device from Medtronic (NYSE:MDT). Several models of the Pipeline Flex embolization device and Pipeline Flex embolization device with Shield Technology were affected, with 8,825 devices recalled in the U.S., having been distributed between April 18, 2019, … [Read more...] about Medtronic’s Pipeline Flex problems expand
Glaukos submits supplemental PMA application for iStent Infinite
Glaukos (NYSE:GKOS) announced today that it submitted a supplemental premarket approval application to the FDA for its iStent Infinite system. San Clemente, California–based Glaukos designed the iStent Infinite trabecular micro-bypass system for use in a standalone procedure to reduce elevated intraocular pressure (IOP) in patients with … [Read more...] about Glaukos submits supplemental PMA application for iStent Infinite
Sinomed launches HT Supreme drug-eluting stent in Europe
Sinomed has completed the first commercial implantation of its HT Supreme drug-eluting stent in Ireland. Faisal Sharif, professor of translational cardiovascular medicine and innovation at the National University of Ireland Galway, successfully performed the first procedure with HT Supreme, a healing-targeted drug-eluting stent designed to treat … [Read more...] about Sinomed launches HT Supreme drug-eluting stent in Europe
Abbott CMO Dr. Nick West touts latest iteration of drug-eluting stent
Abbott executive Dr. Nick West believes the company offers the “best-in-class” drug-eluting stent with its Xience platform. When Abbott (NYSE:ABT) CMO & divisional VP of global medical affairs Dr. Nick West looks back at his use of drug-eluting stents in the early 2000s, all he sees now is innovation. As a practicing interventional … [Read more...] about Abbott CMO Dr. Nick West touts latest iteration of drug-eluting stent
The 18 most innovative medical devices of 2021
The Galien Foundation today announced the nominees for most innovative medical devices for its 15th annual Prix Galien USA Awards. The foundation nominates devices, biotechnology and pharmaceutical products for its annual Prix Galien awards to highlight products designed to improve the human condition. “The Awards Committee is excited to … [Read more...] about The 18 most innovative medical devices of 2021
Endologix wins FDA breakthrough nod for aneurysm sealing system
Endologix announced today that the FDA granted breakthrough device designation for its Chimney endovascular aneurysm sealing system (Chevas). Irvine, Calif.–based Endologix designed its Chevas system as an investigational endovascular abdominal aortic aneurysm (AAA) sealing therapy that combines the Nellix 3.5 endograft with parallel visceral … [Read more...] about Endologix wins FDA breakthrough nod for aneurysm sealing system
Boston Scientific has a serious stent recall problem
The FDA announced today that it deemed Boston Scientific’s recall of its VICI SDS and VICI RDS venous stent systems as Class I, the most serious kind of recall. Marlborough, Mass.-based Boston Scientific’s VICI SDS and VICI RDS venous stent systems treat obstructions and occlusions in narrowed or blocked venous veins. Get the full story at our … [Read more...] about Boston Scientific has a serious stent recall problem
Endologix launches Alto abdominal stent graft in Canada
Endologix (NSDQ:ELGX) today said it launched its Alto abdominal stent graft in Canada following its recent Health Canada approval. The Alto abdominal stent graft system is a polymer-based therapy for abdominal aortic aneurysm (AAA) patients. It uses a low-profile delivery system with a conformable sealing ring that molds in-situ to the patient's … [Read more...] about Endologix launches Alto abdominal stent graft in Canada
iVascular covered stent gains CE mark
iVascular recently announced that it received CE mark approval for its BX-covered stent iCover. Balloon-expanding (BX) covered stents are used to treat arteriosclerotic lesions in renal and iliac arteries and to treat aneurysms and ruptures. The iCover stent adapts to tortuous vessels because of its high flexibility and post-expansion … [Read more...] about iVascular covered stent gains CE mark