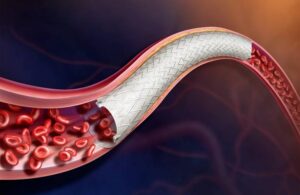
SafeGuard Surgical announced today that the FDA granted breakthrough device designation to its LeakGuard biodegradable stent. Along with the regulatory milestone, the company announced that it completed its Series A funding round. Tom Pepin, principal at Tapper Ventures, led the financing. NFL quarterback Jameis Winston is also one of the first … [Read more...] about SafeGuard Surgical wins FDA breakthrough nod for biodegradable stent backed by NFL QB
Stents
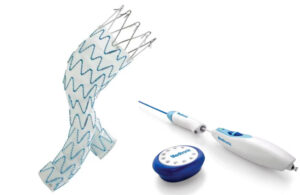
Silk Road Medical launches new transcarotid stent in the U.S.
Silk Road Medical (Nasdaq:SILK) announced today that it launched its tapered EnRoute transcarotid stent system in the U.S. Sunnyvale, California-based Silk Road says this expands upon the prior launch of its EnRoute system by adding additional configurations. It better tailors the transcarotid artery revascularization (TCAR) procedure to the … [Read more...] about Silk Road Medical launches new transcarotid stent in the U.S.
BD initiates IDE trial of stent for treating peripheral arterial disease
BD (NYSE: BDX) announced today that it enrolled the first patient in a trial of its Vascular Covered Stent for treating peripheral arterial disease (PAD). Franklin Lakes, New Jersey–based BD is evaluating the safety and effectiveness of the stent in its AGILITY FDA investigational device exemption (IDE) trial. The self-expanding, low-profile, … [Read more...] about BD initiates IDE trial of stent for treating peripheral arterial disease
Medtronic enrolls first patient in aneurysm repair trial
Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] announced today that it enrolled the first patient in the Hercules trial comparing abdominal aortic aneurysm repair methods. Hercules compares endosuture aneurysm repair (ESAR) to standard endovascular aneurysm repair (EVAR). It looks at these methods in patients who have an abdominal … [Read more...] about Medtronic enrolls first patient in aneurysm repair trial
FDA approves Gore low-profile balloon-expandable endoprosthesis
W.L. Gore & Associates announced today that it received FDA approval for a lower-profile Viabahn VBX balloon-expandable endoprosthesis. FDA approval builds on the company's proven VBX stent graft for treating complex vascular disease. The company says it offers the longest balloon-expandable stent on the market (79 mm) and the widest range … [Read more...] about FDA approves Gore low-profile balloon-expandable endoprosthesis
Olympus completes acquisition of GI stent maker Taewoong Medical
Olympus announced today that it closed its acquisition of Taewoong Medical, a Korea-based medical device manufacturer. Taewoong Medical develops gastrointestinal (GI) metallic stents, among other offerings. Olympus announced its intent to acquire the company for $370 million in February 2023. The deal includes $255.5 million upfront, with up to … [Read more...] about Olympus completes acquisition of GI stent maker Taewoong Medical
Gore enrolls first patients in expandable stent graft trial
W.L. Gore & Associates announced today that it enrolled the first patients in a trial evaluating its Viabahn VBX stent graft. The study compares Gore’s balloon-expandable endoprosthesis to bare metal stenting for patients with complex iliac occlusive disease. It aims to inform practice guidelines around the best modalities suited for … [Read more...] about Gore enrolls first patients in expandable stent graft trial
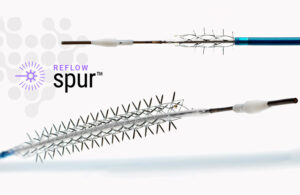
Reflow Medical wins CE mark for temporary spur stent
Reflow Medical announced today that it has received a CE mark in the EU for its Bare Temporary Spur Stent System. The system treats de novo or restenotic lesions in the infrapopliteal arteries, followed by a commercially available drug-coated balloon, to enhance drug absorption. The goal is to provide stent-like results while leaving no metal … [Read more...] about Reflow Medical wins CE mark for temporary spur stent
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
Micro Medical Solutions completes enrollment in vascular stent study
Micro Medical Solutions announced today that it completed enrollment in a U.S. pivotal clinical study of its MicroStent system. Wilmington, Massachusetts-based Micro Medical set up its study to evaluate MicroStent’s safety and effectiveness. The study pits the stent against the current standard of care, percutaneous transluminal angioplasty … [Read more...] about Micro Medical Solutions completes enrollment in vascular stent study