Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] today announced the first commercial use of the Liberant thrombectomy system. Liberant is indicated for the removal of fresh, soft emboli or thrombi from the vessels of the peripheral arterial and venous systems. Medtronic said it expands its portfolio of treatment options for … [Read more...] about Medtronic reports first commercial use of Liberant thrombectomy system
Technologies & Devices
Pulse Biosciences to evaluate PFA in treating thyroid cancer
Pulse Biosciences (Nasdaq:PLSE) today announced a collaboration aimed at using its pulsed field ablation (PFA) technology for thyroid cancer treatment. The Hayward, California-based company entered into a research collaboration with the University of Texas MD Anderson Cancer Center. They aim to examine the use of the nPulse Vybrance percutaneous … [Read more...] about Pulse Biosciences to evaluate PFA in treating thyroid cancer
Conmed to exit gastroenterology product lines, transfer Gore Viabil rights to Olympus
Conmed announced that it plans to exit its gastroenterology product lines as part of a broader portfolio optimization strategy. Part of the exit includes the end of the company's distribution agreement with W.L. Gore & Associates related to the Gore Viabil biliary endoprosthesis. That deal dates back to 2006. Olympus and Gore announced in … [Read more...] about Conmed to exit gastroenterology product lines, transfer Gore Viabil rights to Olympus
Boston Scientific gets European nod for Farapoint catheter
Boston Scientific announced recently that it received CE mark for its Farapoint pulsed field ablation (PFA) catheter. The regulatory nod, earned last week, allows the Marlborough, Massachusetts-based company to bring the newest PFA catheter in its electrophysiology portfolio to the European market. Farapoint treats right atrial flutter (AFL), … [Read more...] about Boston Scientific gets European nod for Farapoint catheter
Atraverse wins FDA clearance for left heart access device
Atraverse Medical announced today that it received FDA clearance for its fully integrated Hotwire transseptal access system. Hotwire is a novel radiofrequency guidewire left-heart access device. It enables zero exchange left-heart access while acting as a rail for catheter-based therapy systems. Steven Mickelsen, a pioneer in the pulsed field … [Read more...] about Atraverse wins FDA clearance for left heart access device
Procyrion adds former Silk Road Medical exec as new CCO
Procyrion announced today that it appointed Andrew Davis as its new chief commercial officer (CCO), effective immediately. Houston-based Procyrion develops a catheter-deployed percutaneous mechanical circulatory support (pMCS) device called Aortix. The company designed the device to treat patients with acute decompensated heart failure (ADHF) … [Read more...] about Procyrion adds former Silk Road Medical exec as new CCO
Xeltis secures $55.1M to support aXess commercialization
Xeltis announced today that it secured €47.5 million ($55.1 million) in financing to support its vascular access device. The funding includes €37.5m ($43.5 million) in funding from the EIB, supported under the European Commission’s Invest EU program, plus €10 million ($11.6 million) from existing investors, including EQT and … [Read more...] about Xeltis secures $55.1M to support aXess commercialization
Shifamed’s Akura Medical closes $53M Series C
Akura Medical, a Shifamed portfolio company, announced today that it completed the first close of its Series C financing, raising $53 million. Los Gatos, California-based Akura plans to use funds to support development activities for its Katana thrombectomy system and the NavIQ quantification software. It also aims to complete enrollment in its … [Read more...] about Shifamed’s Akura Medical closes $53M Series C
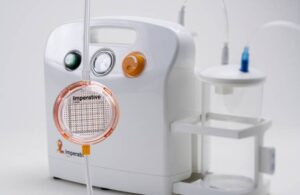
Imperative Care reports positive results for next-gen stroke aspiration approach
Imperative Care announced new real-world data evaluating ADAPT 2.0, a next-generation approach to stroke treatment. ADAPT (A Direct Aspiration First Pass Technique) 2.0 combines 0.088” intracranial access, asymmetric aspiration, and the continuous dual aspiration technique (CDAT) to provide fast and effective clot removal. Campbell, … [Read more...] about Imperative Care reports positive results for next-gen stroke aspiration approach
CMS adds reimbursement for cardiac ablation in ASCs
The Centers for Medicare and Medicaid Services (CMS) has provided a major reimbursement update for cardiac ablation technologies. In its 2026 Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Payment System final rule, CMS added cardiac catheter ablation procedures to its ASC-covered procedures list. This … [Read more...] about CMS adds reimbursement for cardiac ablation in ASCs