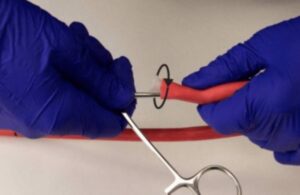
FastWave Medical has completed the first human procedures with its Sola coronary laser intravascular lithotripsy system. The company said that these initial procedures marked the start of its multi-center feasibility study evaluating the platform’s safety and performance. [Scott Nelson, FastWave Medical’s co-founder and CEO, will participate in a … [Read more...] about FastWave Medical reports first-in-human use of coronary laser IVL system
Technologies & Devices
Terumo Neuro launches Sofia Flow 88 for stroke care, expanding aspiration catheter line
Terumo Neuro (formerly MicroVention) has launched the Sofia Flow 88 large-bore aspiration catheter, designed for supporting clot removal in stroke procedures. The catheter features a 0.088 in. inner diameter and incorporates design enhancements aimed at improving procedural control, vessel safety, and compatibility across aspiration … [Read more...] about Terumo Neuro launches Sofia Flow 88 for stroke care, expanding aspiration catheter line
Reflow Medical wins FDA de novo clearance for Spur stent
Reflow Medical today announced it received FDA de novo clearance for its Spur Peripheral Retrievable Stent System for infrapopliteal arterial disease. The system is cleared for use in de novo or restenotic lesions following predilatation in below-the-knee (BTK) vessels. It combines a self-expanding stent with an integrated balloon catheter on an … [Read more...] about Reflow Medical wins FDA de novo clearance for Spur stent
Terumo begins limited release of Roadsaver carotid stent following FDA nod
Terumo Interventional Systems has announced the early commercial availability of its FDA-approved Roadsaver Carotid Stent System. Roadsaver is a dual-layer micromesh carotid stent designed to reduce embolic risk during carotid artery stenting (CAS) procedures. It is indicated for use in patients with carotid artery stenosis who are at increased … [Read more...] about Terumo begins limited release of Roadsaver carotid stent following FDA nod
CoreMap wins FDA IDE for ablation mapping tech
CoreMap announced that it received FDA investigational device exemption (IDE) to extend its electrophysiology (EP) mapping trial to the U.S. The study aims to evaluate the safety and effectiveness of the CoreMap endocardial EP mapping system in patients with AFib. It looks at both persistent AFib and long-standing persistent AFib. The company says … [Read more...] about CoreMap wins FDA IDE for ablation mapping tech
BD to begin real-world atherectomy registry for PAD
BD [WtwhTicker symbol="BDX"](NYSE: BDX)[/WtwhTicker] announced today that it plans to initiate a patient data registry for its Rotarex atherectomy system. The registry aims to measure real-world outcomes for patients with peripheral artery disease (PAD). XTRACT, a multi-center, single-arm, post-market registry assesses Rotarex's clinical … [Read more...] about BD to begin real-world atherectomy registry for PAD
BD warns on esophagogastric balloon tamponade tubes after death
The FDA issued a notice highlighting a letter issued by BD and its C.R. Bard Urology and Critical Care subsidiary warning of new balloon device instructions. This warning follows one death associated with the device issue. BD sent affected customers a notice updating instructions for all lots of certain esophagogastric balloon tamponade tubes. … [Read more...] about BD warns on esophagogastric balloon tamponade tubes after death
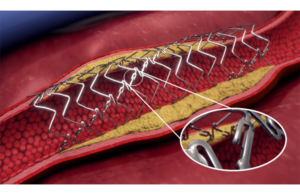
Elixir Medical reports sustained durability with bioadaptor compared to Medtronic stent
Elixir Medical announced three-year results from a large randomized controlled trial of its DynamX coronary bioadaptor system. The 445-patient BIOADAPTOR trial compares DynamX to the standard of care Medtronic Resolute Onyx drug-eluting stent (DES). It took place across 34 centers in Japan, Europe and New Zealand. Data — shared this week at … [Read more...] about Elixir Medical reports sustained durability with bioadaptor compared to Medtronic stent
Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Medtronic announced today that it received CE mark for several expanded indications for its Prevail balloon catheter. The expanded indications for the paclitaxel-coated percutaneous transluminal coronary angioplasty (PTCA) balloon catheter (or drug-coated balloon/DCB) cover the treatment of coronary artery disease (CAD). According to the medtech … [Read more...] about Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Johnson & Johnson MedTech launches ultrasound catheter for imaging in cardiac ablation procedures
Johnson & Johnson MedTech (NYSE: JNJ) announced today that it launched the SoundStar Crystal ultrasound catheter. The company launched the catheter in the U.S. for intracardiac echocardiography (ICE) imaging in cardiac ablation procedures. SoundStar Crystal provides clearer, enhanced image quality compared to other ICE catheters, according … [Read more...] about Johnson & Johnson MedTech launches ultrasound catheter for imaging in cardiac ablation procedures