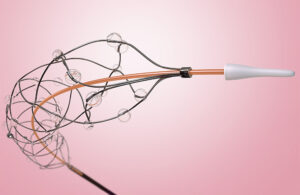
Anaconda Biomed announced today that it received CE mark approval to commercialize its ANA5 funnel catheter in Europe. The company designed ANA5, a next-generation funnel catheter, to optimize mechanical thrombectomy. It maximizes clot capture with its vessel-matching diameter funnel. Simultaneously, it enables antegrade flow arrest and offers the … [Read more...] about Anaconda Biomed wins CE mark for funnel catheter
nitinol
Terumo Neuro launches Sofia Flow 88 for stroke care, expanding aspiration catheter line
Terumo Neuro (formerly MicroVention) has launched the Sofia Flow 88 large-bore aspiration catheter, designed for supporting clot removal in stroke procedures. The catheter features a 0.088 in. inner diameter and incorporates design enhancements aimed at improving procedural control, vessel safety, and compatibility across aspiration … [Read more...] about Terumo Neuro launches Sofia Flow 88 for stroke care, expanding aspiration catheter line
Reflow Medical wins FDA de novo clearance for Spur stent
Reflow Medical today announced it received FDA de novo clearance for its Spur Peripheral Retrievable Stent System for infrapopliteal arterial disease. The system is cleared for use in de novo or restenotic lesions following predilatation in below-the-knee (BTK) vessels. It combines a self-expanding stent with an integrated balloon catheter on an … [Read more...] about Reflow Medical wins FDA de novo clearance for Spur stent
Terumo begins limited release of Roadsaver carotid stent following FDA nod
Terumo Interventional Systems has announced the early commercial availability of its FDA-approved Roadsaver Carotid Stent System. Roadsaver is a dual-layer micromesh carotid stent designed to reduce embolic risk during carotid artery stenting (CAS) procedures. It is indicated for use in patients with carotid artery stenosis who are at increased … [Read more...] about Terumo begins limited release of Roadsaver carotid stent following FDA nod
Surmodics launches Pounce XL thrombectomy system
Surmodics (Nasdaq:SRDX) announced today that it commercially launched the Pounce XL thrombectomy system for clot removal. Pounce XL joins the larger suite of Pounce thrombectomy systems. They offer rapid endovascular removal of acute or chronic clot from peripheral arteries. Eden Prairie, Minnesota-based Surmodics designed Pounce to enable … [Read more...] about Surmodics launches Pounce XL thrombectomy system
Second Heart Assist says Whisper device caused less hemolysis compared to Abiomed Impella CP
Second Heart Assist today announced data from a study highlighting the performance of its Whisper device against a competitive heart pump. The study showed that Whisper — a next-generation percutaneous mechanical circulatory support (pMCS) system — caused significantly less hemolysis than the widely used Johnson & Johnson MedTech/Abiomed … [Read more...] about Second Heart Assist says Whisper device caused less hemolysis compared to Abiomed Impella CP
Stryker’s Inari Medical launches Artix thrombectomy system
Inari Medical — now part of Stryker — announced that it launched its Artix thrombectomy system for arterial thrombectomies. The company built Artix for the distinct needs of the peripheral arterial system, according to a news release. It marks Inari's inaugural entry into the arterial space. The combined aspiration plus mechanical … [Read more...] about Stryker’s Inari Medical launches Artix thrombectomy system
CereVasc study data backs eShunt system
CereVasc today announced initial results from a pilot clinical study supporting the use of its eShunt system. Boston-based CereVasc designed eShunt for treating normal pressure hydrocephalus (NPH). This study evaluates the technology in the treatment of elderly patients with NPH. eShunt has FDA breakthrough device designation, which it picked up … [Read more...] about CereVasc study data backs eShunt system
FDA approves Merit Medical’s cell-impermeable endoprosthesis
Merit Medical Systems (Nasdaq:MMSI) announced today that it received FDA premarket approval for its Wrapsody cell-impermeable endoprosthesis. With approval, the company can begin commercializing the device in the U.S. in 2025. South Jordan, Utah–based Merit Medical designed Wrapsody to extend long-term vessel patency in dialysis patients. It … [Read more...] about FDA approves Merit Medical’s cell-impermeable endoprosthesis
First Look: Flow Medical’s next-generation thrombolysis catheter for pulmonary embolism
Flow Medical is developing a thrombolysis catheter to improve the treatment of blood clots in pulmonary embolism patients. The Chicago-based startup designed its minimally invasive system with a novel nitinol scaffold that perforates blood clots and infuses them with tissue plasminogen activator (TPA), a thrombolytic drug commonly referred to as … [Read more...] about First Look: Flow Medical’s next-generation thrombolysis catheter for pulmonary embolism