Orchestra BioMed (Nasdaq:OBIO) announced today that it entered into a termination and right of first refusal agreement with Terumo. The agreement relates to the New Hope, Pennsylvania–based company's Virtue Sirolimus AngioInfusion Balloon (SAB). It supersedes and terminates a prior distribution agreement between the companies and grants Terumo a … [Read more...] about Orchestra BioMed, Terumo terminate drug-eluting balloon distro deal in $30M agreement
Terumo
Terumo enters U.S. imaging market with FDA nod for dual-sensor system
Terumo Interventional Systems announced today that it received FDA 510(k) clearance for its OpusWave dual-sensor imaging system. OpusWave features the DualView imaging catheter, which received FDA 510(k) clearance alongside the entire system. The platform combines optical frequency domain imaging (OFDI) and intravascular ultrasound (IVUS). It aims … [Read more...] about Terumo enters U.S. imaging market with FDA nod for dual-sensor system
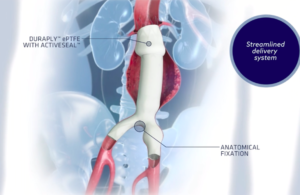
Terumo begins limited release of Roadsaver carotid stent following FDA nod
Terumo Interventional Systems has announced the early commercial availability of its FDA-approved Roadsaver Carotid Stent System. Roadsaver is a dual-layer micromesh carotid stent designed to reduce embolic risk during carotid artery stenting (CAS) procedures. It is indicated for use in patients with carotid artery stenosis who are at increased … [Read more...] about Terumo begins limited release of Roadsaver carotid stent following FDA nod
Terumo Neuro launches new stroke catheter in the U.S.
Terumo Neuro (formerly MicroVention) announced that it launched its Sofia 88 neurovascular support catheter in the U.S. Sofia 88 expands the company's stroke portfolio with a new large-bore catheter engineered for reliability, flexibility and physician control. The company says its launch builds upon the proven performance and legacy of the … [Read more...] about Terumo Neuro launches new stroke catheter in the U.S.
Terumo launches R2P NaviCross peripheral support catheter in US
Terumo is further boosting its radial-to-peripheral portfolio with the U.S. launch of its R2P NaviCross peripheral support catheter. The Japanese medtech giant's Terumo Interventional Systems division designed the R2P NaviCross catheter for optimized performance in R2P procedures, which involve accessing the radial artery around the wrist in … [Read more...] about Terumo launches R2P NaviCross peripheral support catheter in US
MicroVention launches new stent in the U.S.
Terumo subsidiary MicroVention announced that it began the U.S. launch of its LVIS EVO intraluminal support device. The device is now available in the U.S. for the treatment of wide-neck intracranial aneurysms. Aliso Viejo, California-based MicroVention first launched the device in Europe in 2019, selling more than 12,000 units … [Read more...] about MicroVention launches new stent in the U.S.
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts