This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier.
And five catheter-based devices were named among the most innovative technology of the year in the Galien Foundation’s Prix Galien awards. Those devices included Boston Scientific’s Baylis Medical VersaCross catheter, BD’s PureWick female external catheter, BD’s Venovo venous stent system, Boston Scientific’s Eluvia drug-eluting vascular stent system and Edwards Lifesciences’ Sapien 3 transcatheter pulmonary valve system with Alterra adaptive pre-stent,
Looking back at last year’s catheter coverage at Medical Tubing + Extrusion, we analyzed the most popular catheter innovations of 2021 based on reader interest. The list includes devices from other medtech giants like Medtronic and Abbott, as well as smaller companies like Bendit Technologies and Imperative Care and a few university-backed research teams.
Here are the top 10 catheter-based innovations that dominated the news on Medical Tubing + Extrusion in 2022.
 The Portico with FlexNav TAVR system [Image courtesy of Abbott]
The Portico with FlexNav TAVR system [Image courtesy of Abbott]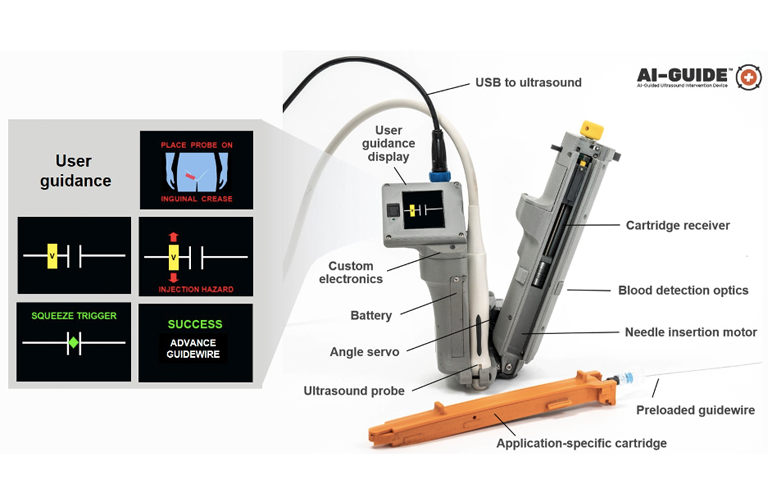 MIT's handheld surgical robotic platform [Image courtesy of the Massachusetts Institute of Technology]
MIT's handheld surgical robotic platform [Image courtesy of the Massachusetts Institute of Technology]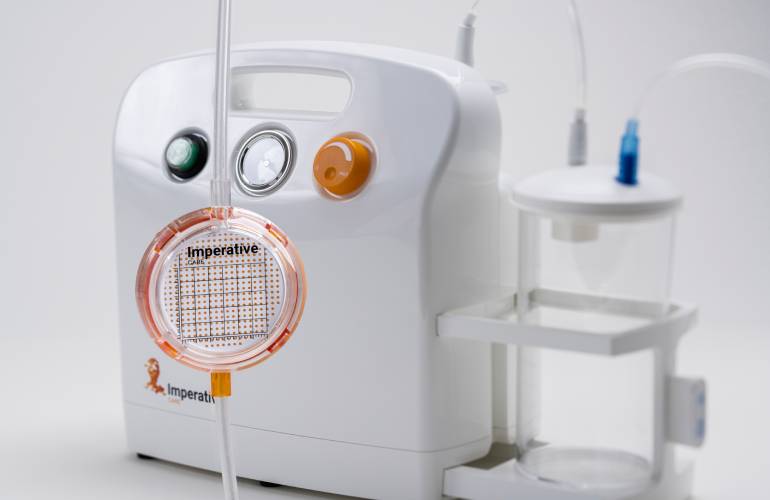 Imperative Care's Zoom POD [Image from Imperative Care]
Imperative Care's Zoom POD [Image from Imperative Care]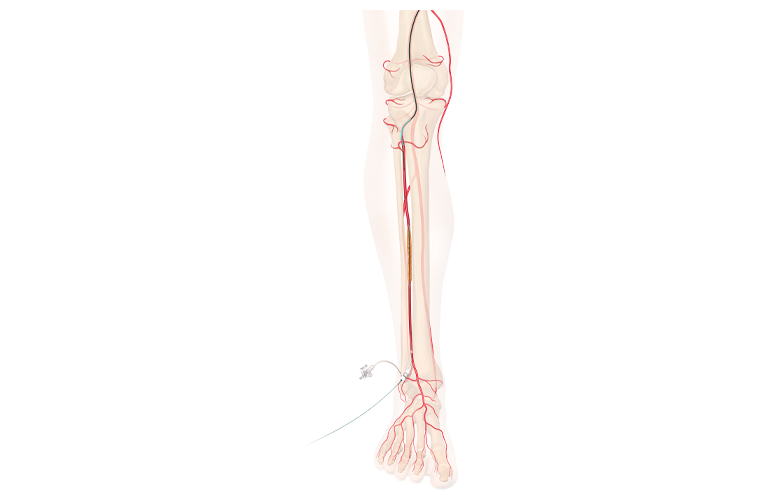 Cook Medical CLTI treatment below-the-knee [Image courtesy of Cook Medical]
Cook Medical CLTI treatment below-the-knee [Image courtesy of Cook Medical] Johnson & Johnson Biosense Webster Octaray mapping catheter with Trueref technology [Image from Biosense Webster]
Johnson & Johnson Biosense Webster Octaray mapping catheter with Trueref technology [Image from Biosense Webster]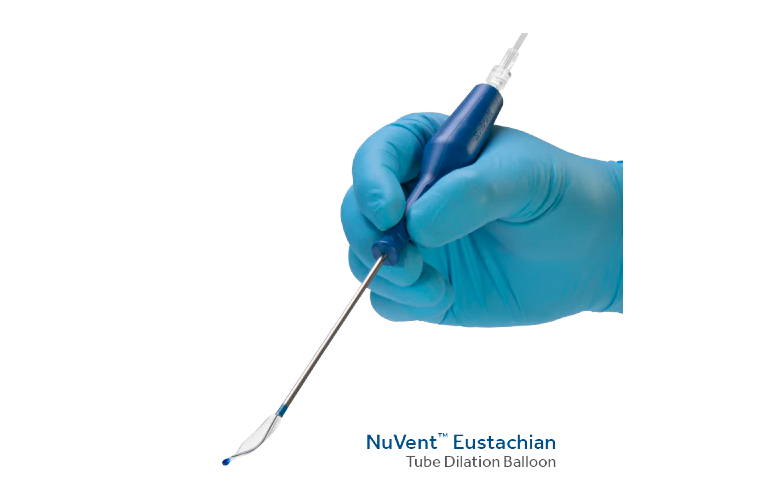 Medtronic's NuVent eustachian tube dilation balloon [Image courtesy of Medtronic]
Medtronic's NuVent eustachian tube dilation balloon [Image courtesy of Medtronic] Variable stiffness catheter that can transition between soft and rigid states. [Image courtesy of EPFL]
Variable stiffness catheter that can transition between soft and rigid states. [Image courtesy of EPFL] Freezor cardiac cryoablation catheters for pediatric atrioventricular nodal reentrant tachycardia. [Image courtesy of Medtronic]
Freezor cardiac cryoablation catheters for pediatric atrioventricular nodal reentrant tachycardia. [Image courtesy of Medtronic] Radiant balloon-expandable stent. [Image from Medtronic]
Radiant balloon-expandable stent. [Image from Medtronic] Bendit21 steerable microcatheter. [Image courtesy of Bendit Technologies]
Bendit21 steerable microcatheter. [Image courtesy of Bendit Technologies](Click + symbol to expand)
Abbott is looking to compete in the transcatheter aortic valve replacement (TAVR) market. The company won FDA approval for its Portico with FlexNav TAVR system in 2021 and the technology behind the device innovates how the valve can be delivered, according to Santosh Prabhu, divisional VP, product development at Abbott Structural Heart.
The Portico valve has a self-expanding design with intra-annular (within the native valve) leaflets. The Portico’s creators designed its structure to provide optimal blood flow when placed inside a patient’s natural valve. It also preserves access to the critical coronary arteries for future interventions.
FlexNav employs strategic use of hydrophilic coatings on specific areas of the catheter to provide lubricity through to the vasculature. The device’s low profile drives the size of the catheter down to 14 Fr. An integrated sheath accommodates the catheter’s size while a stability layer helps take the guesswork out of TAVR deployment. Prabhu also mentioned better benchtop testing that made the Portico delivery system so innovative.
AI-GUIDE is designed with custom-built algorithms and integrated robotics that can be paired with most commercial portable ultrasound devices. To operate the handheld surgical robot, a user places it on a patient’s body near where the thigh meets the abdomen and a targeting display guides the user to the correct location and instructs them to push a button that precisely inserts the needle into the vessel, the researchers said.
The device verified that the needle has penetrated the blood vessel and tells the user to advance a guidewire to guide a catheter into the vessel. The physician can then manually advance the catheter. Once the catheter is securely in the blood vessel, the device withdraws the needle and the user can remove the device. The catheter delivers fluid, medicine and other interventions after it is safely inside the vessel.
Imperative Care launched its Zoom POD aspiration tubing sterile field clot filter device in early 2022. The company designed the system to enable faster time to clot capture during critical mechanical thrombectomy procedures for ischemic stroke patients.
Zoom POD is integrated into the aspiration tubing to shorten the distance from aspiration to filtration while maintaining full aspiration power. Positioned within the sterile field, the physician can immediately see the clot as it is extracted from the patient to quickly confirm clot capture and give the physician more control over the mechanical thrombectomy procedure.
Cook Medical won FDA breakthrough designation for its drug-eluting stent for below-the-knee procedures. The company designed the stent to treat patients who have chronic limb-threatening ischemia (CLTI).
The drug-eluting stent is not yet commercially available, but the breakthrough designation gives the company priority review and interactive and timely communication with FDA during clinical trials and premarket review phases.
Johnson & Johnson’s Biosense Webster launched its Octaray mapping catheter with TRUEref technology in September. Biosense Webster designed Octaray for mapping cardiac arrhythmias, including AFib. It is powered by the company’s Carto 3 Version 7 system.
The catheter has eight splines with improved electrode spacing options and has shorter and more efficient mapping times compared to its Pentaray catheter.
Octaray maps arrhythmias in any chamber of the heart to quickly capture precise information ahead of catheter ablation procedures.
Medtronic launched its NuVent eustachian tube dilation balloon to treat chronic, obstructive eustachian tube dysfunction in September.
The medtech giant designed the NuVent balloon to allow surgeons to deliver treatment in an outpatient setting. It has a flexible balloon section that creates a customized placement option based on unique patient anatomies.
Medtronic says the balloon improves the ability to restore proper Eustachian tube function in an office-based procedure.
A team of researchers from technical university EPFL and ETH Zurich developed the catheter to make minimally invasive surgical interventions like cardiac arrhythmia treatment, simpler and more effective.
The catheter has variable stiffness that can transition between soft and rigid states. The structure of the catheter makes it suitable for cardiac surgeries at just 2.3 mm in diameter, according to the researchers. The engineers fabricated the prototype using a “dipping technique.”
Designed for remote magnetic navigation, the tip of the catheter has a small magnet that can be navigated through the body by an external magnetic field. Surgeons could then control the catheter’s movement with the help of a joystick. Manual catheters require X-rays to continuously monitor the catheter through a procedure, the researchers said.
Medtronic won expanded approval for its Freezor and Freezor Xtra cardiac cryoablation catheters for pediatric atrioventricular nodal reentrant tachycardia (AVRNT).
Medtronic designed the Freezor and Freezor Xtra catheters as flexible, single-use devices that freeze cardiac tissue and block unnecessary electrical signals in the heart. The focal cryoablation therapy has treated more than 140,000 patients across 67 countries, according to the company.
The expanded approval covers kids ages 2 and up and was supported by results from Medtronic’s ICY-AVNRT trial and multiple pediatric randomized, multi-center studies that demonstrated the safety and effectiveness of treating AVNRT with the devices.
Read more about the Freezor and Freezor Xtra catheters here.
Getinge announced in September a partnership with Medtronic over the Radiant balloon-expandable stent, which received the CE mark.
Radiant is designed for use in chimney endovascular aneurysm repair (ChEVAR) with the Endurant II/IIs stent graft system. It maintains perfusion to renal arteries when used in combination with the stent graft. It is one of the first and only covered stents indicated for such use, according to Medtronic.
Radiant comes from a long-term collaboration between Medtronic and Getinge. The platform leverages Getinge’s Advanta V12 balloon expandable covered stent design. Radiant and Endurant II/IIs combine to offer a standardized, on-label, off-the-shelf solution for short-neck, juxtarenal abdominal aortic aneurysms (AAA).
Bendit Technologies won FDA 510(k) clearance for its Bendit21 microcatheter. It is indicated for use in the treatment of the neuro, peripheral and coronary vasculature.
Bendit21 is a steerable microcatheter equipped with a steerable distal tip and controlled by a steering slider on the proximal steering handle. Physicians can bi-directionally rotate the tip by turning a torque knob on the steering handle, according to the company. Bendit Technologies designed the microcatheter to enable navigation with or without guidewires through all vasculatures.
Bendit21 was granted clearance several months after the successful first use of the neurocatheter in the U.S.
