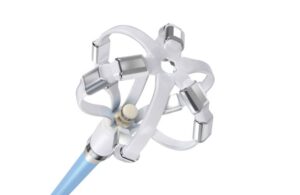
Johnson & Johnson MedTech today announced the completed enrollment of the pilot phase of a clinical trial for its Omnypulse platform. The OMNY-AF clinical trial evaluates the investigational platform for the treatment of symptomatic paroxysmal AFib in the U.S. and Australia. The single-arm, multi-center trial completed the pilot phase … [Read more...] about Johnson & Johnson completes pilot enrollment in Omnypulse trial
Johnson & Johnson
Johnson & Johnson reports first commercial Varipulse PFA use in Canada
Johnson & Johnson MedTech [WtwhTicker symbol="JNJ"](NYSE: JNJ)[/WtwhTicker] today announced the first completed commercial case with its Varipulse platform in Canada. The company said in a post on LinkedIn that doctors at the Ottawa Heart Institute completed the first commercial case with Varipulse. Johnson & Johnson said the procedure … [Read more...] about Johnson & Johnson reports first commercial Varipulse PFA use in Canada
J&J’s Shockwave adds enhanced catheter to U.S. IVL portfolio
Johnson & Johnson MedTech's Shockwave Medical today announced the full U.S. launch of its Shockwave E8 peripheral IVL catheter. Santa Clara, California-based Shockwave launched the intravascular lithotripsy (IVL) catheter following FDA clearance. It designed the catheter to optimize the treatment of patients with calcified femoro-popliteal … [Read more...] about J&J’s Shockwave adds enhanced catheter to U.S. IVL portfolio
J&J’s Biosense Webster completes enrollment in pulsed field ablation trial, earns coverage win in Japan
Johnson & Johnson MedTech’s Biosense Webster announced today that it completed enrollment in its Omny-IRE pulsed field ablation (PFA) trial. The prospective, multi-center, non-randomized trial enrolled 188 patients in Europe and Canada. It evaluates the safety and effectiveness of the company's Omnypulse platform. The J&J unit designed … [Read more...] about J&J’s Biosense Webster completes enrollment in pulsed field ablation trial, earns coverage win in Japan
Biosense Webster reports positive dual-energy ablation results
Johnson & Johnson's Biosense Webster unit today announced positive three-month follow-up results from its SmartfIRE clinical trial. The study evaluated the use of the dual-energy ThermoCool SmartTouch SF catheter. ThermoCool SmartTouch SF is the first dual-energy pulsed field ablation (PFA)/radiofrequency (RF) ablation catheter integrated … [Read more...] about Biosense Webster reports positive dual-energy ablation results
FDA says recall of Cerenovus catheter guide sheaths is serious
The FDA deemed a recall of catheter guide sheaths from Johnson & Johnson’s (NYSE:JNJ) Cerenovus Class I, the most serious kind. Medos International Sàrl, a J&J unit, recalled the Cerenovus Cerebase DA guide sheath and single-use neurovascular guide catheter on Feb. 2, 2024. The company distributed the devices — 1,343 in total — between … [Read more...] about FDA says recall of Cerenovus catheter guide sheaths is serious
J&J’s Acclarent wins FDA nod for pediatric ear tube balloon dilation system
Johnson & Johnson MedTech unit Acclarent announced that it won a new FDA clearance for its Aera Eustachian tube balloon dilation system. While Acclarent still belongs to Johnson & Johnson, Integra Lifesciences is set to buy it for $275 million next year. While the completion of that deal awaits, the company cleared a regulatory hurdle … [Read more...] about J&J’s Acclarent wins FDA nod for pediatric ear tube balloon dilation system
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Johnson & Johnson's Biosense Webster today announced the first completed patient cases in a study of its dual-energy ablation catheter. Biosense Webster designed the ThermoCool SmartTouch SF to deliver both radiofrequency (RF) and pulsed-field ablation (PFA) energy. The SmartPulse pivotal study evaluates the dual-energy system in the … [Read more...] about J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
Biosense Webster: catheter ablation leads to lower heart failure risk in AFib patients
Johnson & Johnson's Biosense Webster today announced data supporting the use of catheter ablation in AFib patients. The study, funded by the Irvine, California-based company, looked at heart failure incidence risks in AFib patients. It compared the use of catheter ablation versus antiarrhythmic drugs (AAD). Looking at non-specific catheter … [Read more...] about Biosense Webster: catheter ablation leads to lower heart failure risk in AFib patients