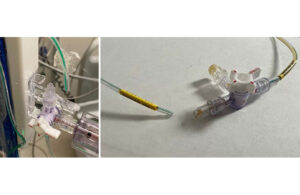
The FDA today designated a Medtronic recall of Duet external drainage and monitoring system catheter tubing as Class I, its most serious level. Medtronic Neurosurgery initiated the recall on Jan. 22. According to the FDA, it involves 45,176 devices distributed from May 3, 2021, to Jan. 9, 2024. The model numbers involved are 46913, 46914, 46915, … [Read more...] about Medtronic has a serious catheter tubing recall
Recalls
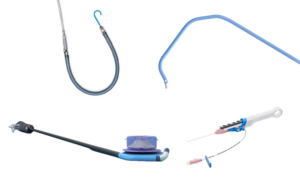
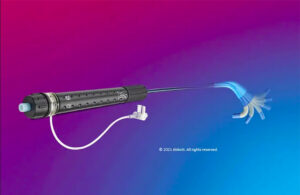
Boston Scientific warns on fragment embolization in some PolarSheath devices
Boston Scientific has issued an urgent field safety notice for some of its PolarSheath steerable sheaths due to the risk of embolization. The company is removing specific batches of PolarSheath steerable sheaths due to a tooling error in manufacturing, which may have caused delimitation of the inner lumen of the sheath shaft in a subset of … [Read more...] about Boston Scientific warns on fragment embolization in some PolarSheath devices
ICU Medical’s Smiths Medical has a major syringe pump recall
The FDA this week said it's designated an ICU Medical and Smiths Medical recall of Medfusion syringe pumps as Class I, the agency's most serious level of medical device recall. The recall involves more than 50,000 Medfusion Model 4000 syringe infusion pumps in the U.S. that were distributed from Nov. 16, 2010, to July 28, 2023. The Medfusion … [Read more...] about ICU Medical’s Smiths Medical has a major syringe pump recall
The worst catheter-based device recalls of 2023
The U.S. saw seven severe catheter-based device recalls in 2023, according to the FDA's medical device recall database. The agency in 2023 tagged 61 medical device recalls as Class I — the most serious level. For comparison, the FDA recalled 60 devices in 2022, of which four were related to catheter-based devices. Last year, the most serious … [Read more...] about The worst catheter-based device recalls of 2023
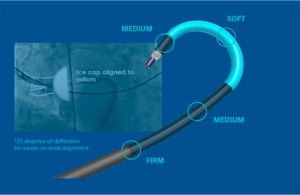
Abbott has a serious recall involving its Amplatzer delivery sheath
The FDA today said an Abbott recall involving its Amplatzer steerable delivery sheath is Class I, its most serious level. Launched in the U.S. in April 2022, the Amplatzer sheath delivers Abbott's Amplatzer Amulet left atrial appendage occluder. Competing against Boston Scientific's Watchman, Amplatzer Amulet provides complete occlusion in order … [Read more...] about Abbott has a serious recall involving its Amplatzer delivery sheath
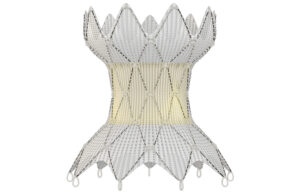
After recall and relaunch, Medtronic wants to go global with its catheter-delivered Harmony valve
Each Medtronic Harmony valve is sewn by hand to attach laser-cut pig tissue to the nitinol that makes this minimally invasive heart implant possible. Medtronic’s Harmony transcatheter pulmonary valve (TPV) design is paying off after engineers solved a delivery catheter recall and relaunched the system this year. The Harmony TPV uses pig tissue, … [Read more...] about After recall and relaunch, Medtronic wants to go global with its catheter-delivered Harmony valve
Olympus corrective action covers laser-compatible bronchoscopes
Olympus is warning health providers about incompatible laser use with bronchoscopes after receiving reports of endobronchial combustion, serious patient injury, and one death. The Japanese medtech giant said on July 3 that its voluntary field corrective action involves 32 BF series endoscope models, 19 of which it distributes in the U.S. The … [Read more...] about Olympus corrective action covers laser-compatible bronchoscopes
Teleflex recall of Arrow catheter system is Class I
The FDA today designated Teleflex's recall of its Arrow Endurance extended dwell peripheral catheter system as Class I, the most serious type of medical device recall. The recall involved 262,016 devices distributed from October 26, 2018, to May 10, 2023, according to the FDA. Teleflex and its Arrow International subsidiary have reported 83 … [Read more...] about Teleflex recall of Arrow catheter system is Class I
Fresenius Kabi recall of Ivenix infusion systems in Class I
The FDA this week said the Fresenius Kabi recall of some Ivenix infusion systems is Class I, the most serious kind. Fresenius Kabi is recalling some Ivenix infusion systems due to a leak in the system that allows fluid to enter the administration set loading area near the air detector. The leak could damage the electrical system and result in … [Read more...] about Fresenius Kabi recall of Ivenix infusion systems in Class I
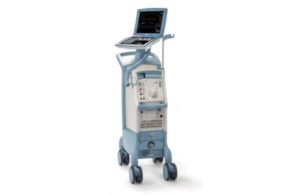
Getinge’s Datascope has its second Class I recall in a month
The FDA issued a notice determining another recall of Getinge subsidiary Datascope’s Cardiosave intra-aortic balloon pumps (IABPs) as Class I, the most serious kind. This recall relates to the Swedish medtech company’s Cardiosave Hybrid and Cardiosave Rescue IABPs. Both devices were subject to a separate Class I recall earlier this month. The … [Read more...] about Getinge’s Datascope has its second Class I recall in a month