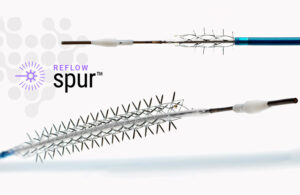
Reflow Medical announced today that it has received a CE mark in the EU for its Bare Temporary Spur Stent System. The system treats de novo or restenotic lesions in the infrapopliteal arteries, followed by a commercially available drug-coated balloon, to enhance drug absorption. The goal is to provide stent-like results while leaving no metal … [Read more...] about Reflow Medical wins CE mark for temporary spur stent
Stents
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
Micro Medical Solutions completes enrollment in vascular stent study
Micro Medical Solutions announced today that it completed enrollment in a U.S. pivotal clinical study of its MicroStent system. Wilmington, Massachusetts-based Micro Medical set up its study to evaluate MicroStent’s safety and effectiveness. The study pits the stent against the current standard of care, percutaneous transluminal angioplasty … [Read more...] about Micro Medical Solutions completes enrollment in vascular stent study
Inari Medical plans to buy LimFlow Medical for up to $415M
Inari Medical today announced a definitive agreement to purchase LimFlow Medical for up to $415 million. The deal comes just weeks after Paris-based LimFlow won FDA premarket approval (PMA) for its breakthrough system to treat chronic limb-threatening ischemia (CLTI). San Jose, California–based Inari said it expects to complete the … [Read more...] about Inari Medical plans to buy LimFlow Medical for up to $415M
The biggest cardiovascular stories from TCT 2023
Every year, some of the biggest names in cardiovascular technologies come together in one place for TCT. This year's 35th edition of the Transcatheter Cardiovascular Therapeutics annual scientific symposium was no different in San Francisco. Usual suspects like Medtronic and Abbott had data on display for a range of products, while other big … [Read more...] about The biggest cardiovascular stories from TCT 2023
Abbott has positive data for Esprit drug-eluting resorbable scaffold
Abbott today announced data supporting its Esprit BTK everolimus-eluting resorbable scaffold system. The company designed Esprit BTK to treat people with chronic limb-threatening ischemia (CLTI), a severe stage of peripheral artery disease (PAD). Abbott said its LIFE-BTK trial met both of its primary safety and effectiveness endpoints. It … [Read more...] about Abbott has positive data for Esprit drug-eluting resorbable scaffold
Cordis applauds Medicare expansion for carotid stenting
Cordis announced today that it supports a new expanded coverage decision from the Centers for Medicare & Medicaid Services (CMS). The expanded CMS coverage widens access to minimally invasive endovascular carotid therapy for patients with carotid artery disease. Cordis develops the Precise Pro Rx carotid stent system for this therapy. In a … [Read more...] about Cordis applauds Medicare expansion for carotid stenting
How LimFlow’s foot-saving system prevents amputations in patients with no other options
LimFlow’s breakthrough system for treating chronic limb-threatening ischemia (CLTI) is the first of its kind approved by the FDA for this severe form of peripheral artery disease (PAD). For CLTI patients who have lost blood flow below their knee and have no other suitable endovascular or surgical treatment options available, the LimFlow System … [Read more...] about How LimFlow’s foot-saving system prevents amputations in patients with no other options
BD enrolls first patient in study of stent graft for treating portal hypertension
BD (NYSE:BDX) announced today that it completed the enrollment of the first patient in a trial of its Liverty TIPS stent graft. Franklin Lakes, New Jersey-based BD's ARCH FDA investigational device exemption (IDE) study evaluates the safety and effectiveness of Liverty. The global, prospective, multi-center, single-arm clinical study looks at … [Read more...] about BD enrolls first patient in study of stent graft for treating portal hypertension
Gore enrolls first patient in Gore ViaFort vascular stent iliofemoral study
Gore this week announced it enrolled the first U.S. patient in its pivotal study for its ViaFort vascular stent. The prospective, non-randomized, multi-center, single-arm study will have a five-year follow-up and will evaluate the investigational Gore ViaFort vascular stent for the treatment of symptomatic iliofemoral venous obstruction. It will … [Read more...] about Gore enrolls first patient in Gore ViaFort vascular stent iliofemoral study