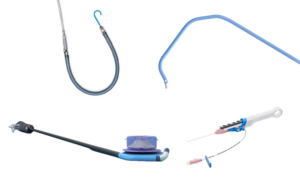
Surmodics (Nasdaq:SRDX) today announced the successful early clinical use of its Pounce LP thrombectomy system. Eden Prairie, Minnesota-based Surmodics designed the LP (low-profile) thrombectomy system for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature. It performs this removal in vessels between 3.5 mm … [Read more...] about Surmodics has positive early results from thrombectomy system
Catheters
The worst catheter-based device recalls of 2023
The U.S. saw seven severe catheter-based device recalls in 2023, according to the FDA's medical device recall database. The agency in 2023 tagged 61 medical device recalls as Class I — the most serious level. For comparison, the FDA recalled 60 devices in 2022, of which four were related to catheter-based devices. Last year, the most serious … [Read more...] about The worst catheter-based device recalls of 2023
FastWave Medical kicks off first-in-human study of its IVL tech
FastWave Medical announced the successful completion of enrollment for its first-in-human study of its peripheral intravascular lithotripsy (IVL) system to treat calcified cardiovascular disease. Dr. Miguel Montero-Baker of Houston Methodist Hospital and the Hope Vascular & Podiatry Clinic and Dr. Venkatesh Ramaiah of HonorHealth Vascular … [Read more...] about FastWave Medical kicks off first-in-human study of its IVL tech
Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Cook Medical recently announced that its Slip-Cath Beacon Tip hydrophilic selective catheter is once again available in the United States and Canada. This reintroduction follows a collaborative effort with medical professionals to refine the product to meet clinical needs better. The Slip-Cath is designed for vascular and non-vascular … [Read more...] about Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
Johnson & Johnson MedTech's Biosense Webster today announced Japanese approval for its Varipulse platform for treating AFib. Varipulse treats symptomatic drug-refractory recurrent paroxysmal AFib using pulsed field ablation (PFA). The platform features the Varipulse catheter, a variable-loop multielectrode catheter, the TruPulse generator … [Read more...] about Biosense Webster wins Japanese approval for Varipulse pulsed field ablation
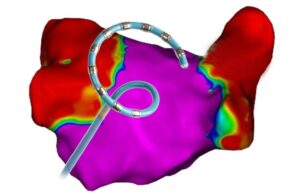
First patients treated using Stereotaxis MAGiC ablation catheter
Stereotaxis today announced the first patients have been successfully treated using its MAGiC magnetic interventional ablation catheter. The MAGiC catheter is a robotically navigated magnetic ablation catheter that performs minimally invasive cardiac ablation procedures. The first procedures were performed in Lithuania in eight patients … [Read more...] about First patients treated using Stereotaxis MAGiC ablation catheter
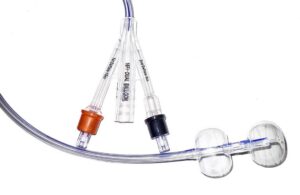
HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical
HR Pharmaceuticals announced that it entered into an exclusive commercial agreement with Poiesis Medical to license catheter technology. The deal centers around Poiesis' dual-balloon catheter, the Duette system. Under the terms, HR Pharmaceuticals has exclusive commercialization rights to Duette in North America. The deal expands HR … [Read more...] about HR Pharmaceuticals licenses dual-balloon catheter tech from Poiesis Medical
Catheter-based innovations we can’t stop thinking about
Catheter-based devices had their year in 2023. Medtech companies set their sights on improving patient care in cardiology, neurology and more. Our previous roundup of the top 10 most-read catheter-based news stories of 2023 wasn't enough for us. Medical Tubing + Extrusion's editors could not stop thinking about several other stories when … [Read more...] about Catheter-based innovations we can’t stop thinking about
The 10 most-read catheter-based innovation stories of 2023
This year was a year of firsts for catheter-based innovations, most notably for renal denervation and pulsed field ablation. ReCor Medical won an FDA panel nod for its renal denervation system in August. The panel voted in favor of the safety and efficacy of its Paradise ultrasound renal denervation (RDN) system for treating hypertension. The … [Read more...] about The 10 most-read catheter-based innovation stories of 2023
FDA clears neurovascular aspiration, access catheters from Perfuze
Perfuze announced today that the FDA cleared its Millipede 070 aspiration catheter and second-generation Millipede 088 access catheter. Galway, Ireland-based Perfuze developed Millipede 070 to address critical unmet needs in ischemic stroke treatment. It aims to remove clots rapidly and safely through a novel, unique catheter. Millipede 070 … [Read more...] about FDA clears neurovascular aspiration, access catheters from Perfuze