Cook Medical recently announced that its Slip-Cath Beacon Tip hydrophilic selective catheter is once again available in the United States and Canada. This reintroduction follows a collaborative effort with medical professionals to refine the product to meet clinical needs better. The Slip-Cath is designed for vascular and non-vascular … [Read more...] about Cook Medical brings Slip-Cath Beacon Tip back to the U.S.
Cook Medical
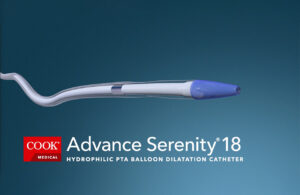
Cook Medical has more hydrophilic PTA balloon catheter options
Cook Medical today announced more sizes and locations for the Advance Serenity hydrophilic PTA balloon catheter product line. It's now possible for U.S. and Canadian interventionalists to use Advance Serenity for below-the-knee and above-the-knee procedures to treat patients with peripheral artery disease (PAD), according to Cook Medical. In … [Read more...] about Cook Medical has more hydrophilic PTA balloon catheter options
Cook Medical, Vizient ink contract for endoscopy devices
Cook Medical announced today that it received a contract with Vizient covering the company's endoscopy devices. The contract enables Cook to continue offering its endoscopy devices at negotiated pricing terms to Vizient members. “We are honored to have been chosen as an Endoscopy contracted supplier with Vizient," said Rick Simms, Cook … [Read more...] about Cook Medical, Vizient ink contract for endoscopy devices
The top 10 catheter innovation news stories of 2022
This year was a big one for catheter innovation as medtech companies large and small received regulatory approvals and researchers developed catheters that could soon make navigating the vasculature system easier. The numerous innovations weren't just limited to adults. One company launched a cryoablation catheter for children as young as 2 … [Read more...] about The top 10 catheter innovation news stories of 2022
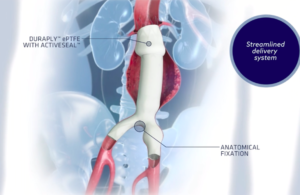
Cook Medical wins FDA breakthrough designation for Thoraco+ endovascular system
Cook Medical today announced that its Zenith Thoraco+ endovascular system received FDA breakthrough device designation. Thoraco+ is the company's next-generation endovascular graft that is indicated for the endovascular treatment of patients with thoracoabdominal aortic aneurysms. “We are excited to receive an FDA breakthrough device … [Read more...] about Cook Medical wins FDA breakthrough designation for Thoraco+ endovascular system
Cook Medical wins FDA breakthrough designation for new drug-eluting stent
Cook Medical recently announced that it received FDA breakthrough designation for its drug-eluting stent for below the knee. Bloomington, Indiana-based Cook Medical designed the stent to treat patients who have chronic limb-threatening ischemia (CLTI). "CLTI is a debilitating disease of growing prevalence around the globe and this is Cook … [Read more...] about Cook Medical wins FDA breakthrough designation for new drug-eluting stent
The top stories from Medical Tubing + Extrusion in 2021
The medical device industry as a whole experienced a handful of innovations in 2021, but Medical Tubing + Extrusion's reader took a special interest in two catheter-based innovations last year. Here are our most popular articles of 2021: Links to articles: Cook Medical wins FDA breakthrough device designation for next-gen … [Read more...] about The top stories from Medical Tubing + Extrusion in 2021
Cook Medical issues voluntary recall for transseptal needles, transseptal needles with catheter
Cook Medical this week announced a global voluntary recall of its Transseptal Needle and Transseptal Needle with catheter. Bloomington, Indiana-based Cook Medical issued the recall due to complaints of rust on the products. The use of both products could result in increased procedural time and inflammatory reactions, including systemic reactions … [Read more...] about Cook Medical issues voluntary recall for transseptal needles, transseptal needles with catheter
Cook Medical warns on some Flexor Check-Flo introducer sheaths
Cook Medical recently issued a warning letter regarding an issue with its Flexor Check-Flo introducer catheters. Bloomington, Indiana-based Cook Medical's field safety corrective action informs users that some of its introducer sheaths may be manufactured incorrectly and that the radiopaque marker band may be located just below the Check-Flo … [Read more...] about Cook Medical warns on some Flexor Check-Flo introducer sheaths
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts